Figure 3.
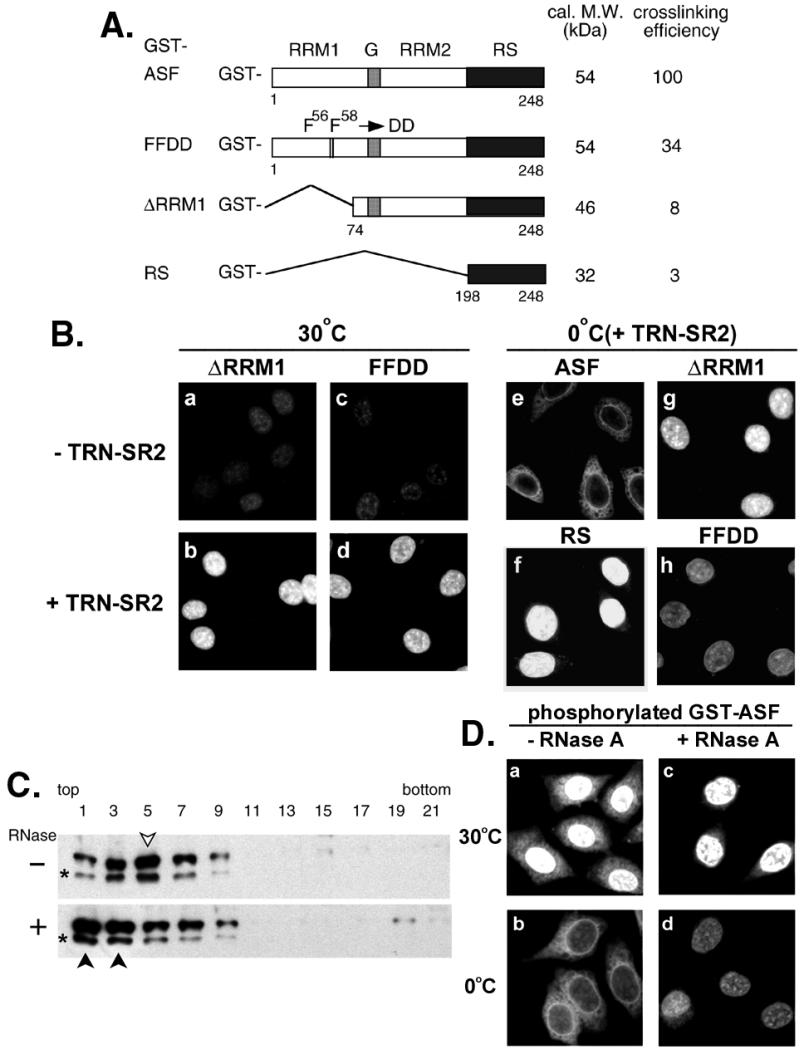
RNA-binding capacity of SR proteins correlates with their temperature sensitivity to nuclear import. (A) Schematic representation of the GST fusions with ASF, FFDD, ΔRRM1, and RS domain only. Calculated molecular mass (in kDa) of unphosphorylated fusion proteins and relative UV-crosslinking efficiency of phosphorylated proteins are indicated, respectively (Right). G represents the glycine-rich hinge of ASF. (B) (a–d) Nuclear import of phosphorylated GST-ΔRRM1 and GST-FFDD was assayed at 30°C in the mock E. coli lysate or in lysate containing TRN-SR2. (e–h) Phosphorylated GST fusion SR proteins (as indicated) were subjected to nuclear import assay in the TRN-SR2-containing lysate on ice. (C) Phosphorylated GST-ASF was incubated with E. coli lysate in the absence (−) or presence (+) of RNase A under the import conditions. The reaction mixtures were sedimented on 10–30% glycerol gradient, followed by Western blot analysis by using anti-GST antibodies. GST-ASF peak fractions are indicated by arrowheads (open for mock-treated and closed for RNase-treated). Asterisks represent partially degraded GST-ASF. (D) Nuclear import of phosphorylated GST-ASF was assayed in the RNase A-treated (+RNase A) or mock-treated (−RNase A) TRN-SR2-containing extract at 30°C or on ice.
