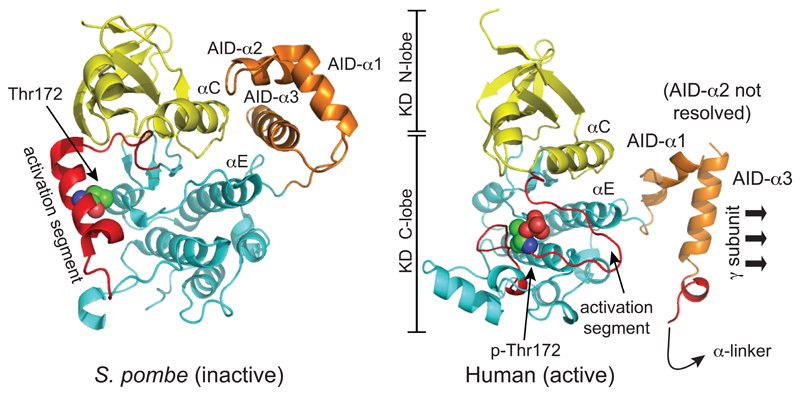
Figure 3. Comparison of structure of the inactive KD:AID fragment from S. pombe, and the active KD:AID fragment from human α2, showing the dramatic change in the position of the AID.
The models were created with McPymol using PDB entries 3H4J and 4CFE (although the latter was a complete heterotrimer, only the KD and AID are shown). The kinase domains are viewed from approximately the same angle, so that the change in the orientation of the AID is more obvious. The N- and C-lobes of the kinase domain are in yellow and cyan respectively, except that the activation segments within the C-lobe (which have markedly different conformations in the two structures) are in red. The AIDs are in orange, with their three α-helices labeled AID-α1 to -α3 (AID-α2 was poorly resolved in the human structure). In the inactive S. pombe structure, AID-α3 interacts with αC (C-helix) from the N-lobe and αE (E-helix) from the C-lobe, whereas in the active human structure it interacts mainly with the γ subunit (not shown) instead.