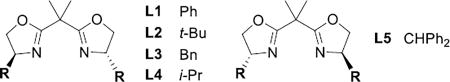
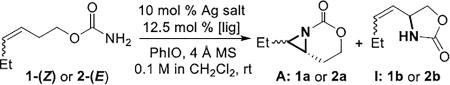
Table 1.
Initial optimization of the asymmetric aziridination.
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entry | substrate | Ag salt | ligand | yield A[a] | yield I[a,b] | ee A |
| 1 | 1-(Z) | AgClO4 | L1 | 46% | 7% | 16% |
| 2 | 1-(Z) | AgClO4 | L2 | 89% | 10% | 82% |
| 3 | 1-(Z) | AgClO4 | L3 | 77% | 6% | 45% |
| 4 | 1-(Z) | AgClO4 | L4 | 81% | 11% | 23% |
| 5 | 1-(Z) | AgClO4 | L5 | 91% | 9% | (−)80% |
|
| ||||||
| 6 | 1-(Z) | AgOAc | L2 | 29% | 16% | 6% |
| 7 | 1-(Z) | AgOTf | L2 | 63% | 10% | 67% |
| 8 | 1-(Z) | AgOTf | L5 | 86% | 6% | (−)81% |
| 9 | 1-(Z) | AgBF4 | L2 | 92% | 8% | 71% |
| 10 | 1-(Z) | AgBF4[c] | L2 | 55% | 5% | 76% |
| 11 | 1-(Z) | AgBF4 | L5 | 74% | 10% | (−)67% |
| 12 | 1-(Z) | AgPF6 | L2 | 69% | 6% | 71% |
| 13 | 1-(Z) | AgSbF6 | L2 | 83% | 5% | 79% |
|
| ||||||
| 14 | 2-(E) | AgBF4 | L2 | 73% | 11% | 83% |
| 15 | 2-(E) | AgPF6 | L2 | 73% | 13% | 79% |
| 16 | 2-(E) | AgSbF6 | L2 | 79% | 8% | 83% |
| 17 | 2-(E) | AgClO4 | L2 | 79% | 17% | 85% |
| 18 | 2-(E) | AgClO4 | L5 | 90% | 10% | (−)84% |
NMR yield using mesitylene as internal standard.
I: C-H insertion byproduct.
NaBARF (BARF = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate) was employed as an additive.