Table 2.
Scope of the asymmetric aziridination.
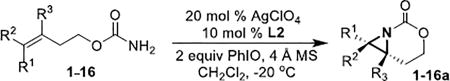
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | substrate | product | entry | substrate | product |
| 1 | 1-(Z) |
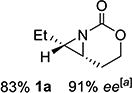
|
9 | 9-(E) |
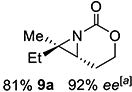
|
| 2 | 2-(E) |
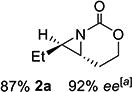
|
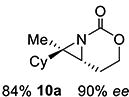
10 | 10-(E) |
|
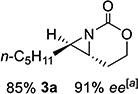
| 3 | 3-(E) |
|
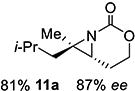
11 | 11-(E) |
|
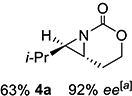
| 4 | 4-(E) |
|
12 | 12-(E) |
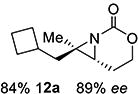
|
| 5 | 5-(E) |
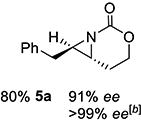
|
13 | 13-(E) |
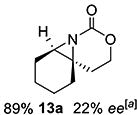
|
| 6 | 6-(E) |
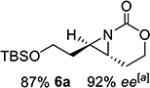
|
14 | 14-(Z) |
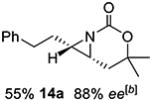
|
| 7[c] | 7-(E) |
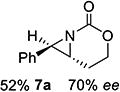
|
15 | 15-(Z) |
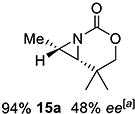
|
| 8 | 8-(E) |
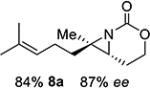
|
16 | 16 allene |
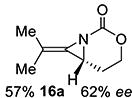
|
ee was determined after ring-opening of aziridine with NaI.
ee after one recrystallization.
Reaction was run in C6H6 at room temperature.
