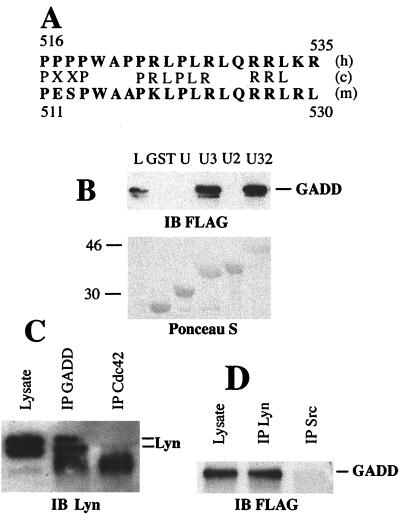
Figure 2.
GADD34–Lyn interaction in vitro and in mammalian cells. (A) Alignment of human GADD34 (h) and mouse MyD116 (m) proline-rich clusters with Lyn SH3 binding site consensus sequence (c). Single-letter amino acid code is used. Residue numbers are shown. (B) GST pull-down assay with GST or GST fusions with unique (U), unique SH3 (U3), unique SH2 (U2), and unique SH3-SH2 (U32) domains of Lyn, and lysates of HEK293 cells transiently transfected with pFLAG-CMV2-GADD34. Proteins from lysate bound to GST fusion beads were analyzed by using immunoblot (IB). (Upper) Immunoblot probed with the anti-FLAG antibody. (Lower) Blot stained with Ponceau S to show equal load of GST fusions. L, total cell lysate lane shown to indicate position of FLAG-GADD34. Size markers on the left are in kilodaltons. The minor band migrating slightly faster than FLAG-GADD34 is a result of partial degradation. (C) Immunoblot of Daudi cell lysate, anti-GADD34 (IP GADD), and control (IP Cdc42) immunoprecipitates probed with the monoclonal anti-Lyn antibody H-6. The diffuse bands at 46 kDa in immunoprecipitate lanes are because of the presence of crossreacting Ig heavy chain. Position of Lyn doublet is indicated. (D) Immunoblot of the lysate of 293 cells transfected with pFlag-CMV2-GADD34, anti-Lyn, and anti-Src immunoprecipitates probed with the anti-GADD34 antibody. Position of GADD34 is indicated.