Table 2.
IC50 values of substituted 2-phenyl-N-(3,5-dichlorophenyl)-2-oxoacetohydrazonoyl cyanides for inhibiting EPAC1/2 GEF activity.
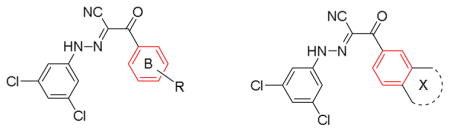
| |||
|---|---|---|---|
| Entry | R or X | Rap1b-bGDP EPAC1 IC50 (μM)a | Rap1b-bGDP EPAC2 IC50 (μM) |
| 10 | 4-tert butyl | 5.4 ± 0.7 | 2.5 ± 0.6 |
| 22 | 4-Cl | >300 | NDb |
| 23 | 3-Cl | >300 | ND |
| 24 | 4-OCF3 | 40.9 ± 6.8 | ND |
| 25 | 4-CO2Me | 29.3 ± 6.2 | ND |
| 26 | 4-Ph | >300 | ND |
| 27 | 3,4-di OMe | >300 | ND |
| 28 | 4-Piperidin-1-ylmethyl | 39.4 ± 4.7 | ND |
| 29 | 4-Morpholinomethyl | 14.6 ± 5.7 | ND |
| 30 | 4-Cyclohexane | 32.4 ± 9.5 | ND |
| 31 |
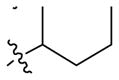
|
3.6 ± 0.3 | 1.2 ± 0.1 |
| 32 |
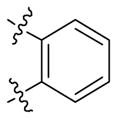
|
11.3 ± 1.4 | 2.5 ± 0.5 |
| 33 |
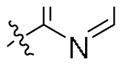
|
19.6 ± 6.7 | ND |
The values are the mean ± SE.
ND: not determined.
