Abstract
Whether diabetes after donation is associated with an accelerated GFR decay in the remaining kidney has not been studied. We determined the incidence of diabetes in kidney donors, compared GFR change over time in diabetic to non-diabetic donors, and also the impact of DM on the development of proteinuria, hypertension and ESRD. Of the 4014 donors, 309(7.7%) developed diabetes at a median age of 56.0 years and after a median of 18 years post-donation. The difference in annual eGFR change between diabetic and non-diabetic donors in the seven years prior to DM development was −0.08 ml/min/year; p=0.51. After DM development, the difference was −1.10 ml/min/year for diabetics with hypertension and proteinuria, p<0.001; −0.19 for diabetic donors with hypertension but no proteinuria, p=0.29; −0.75 ml/min/year for diabetic donors with proteinuria but no hypertension, p=0.19 and −0.09 ml/min/year for diabetic donors without proteinuria or hypertension, p=0.63. When DM was considered as a time-dependent covariate, it was associated with the development of proteinuria (HR 2.65 (95%CI 1.89, 3.70), p<0.001) and hypertension (HR 2.19 (95%CI 1.74, 2.75), p<0.001). It was not, however, associated with ESRD. eGFR decline after DM development exceeds that of non-diabetic donors only in those with concomitant proteinuria and hypertension.
Introduction
Hyperfiltration occurs in both diabetes mellitus (DM) and following reduction in renal mass (1,2). Post-donation DM and its impact on the development of proteinuria, hypertension and reduced glomerular filtration rate (GFR) in kidney donors have not been sufficiently addressed. This is important as a substantial proportion of donors donate to family members with DM, a disease which has a strong genetic component. Moreover, diabetes in the setting of reduced renal mass offers a unique opportunity to quantify the impact of hyperfiltration in those without intrinsic kidney disease. Suggestive evidence from kidney transplant recipients who receive a transplant for DM and our previous short term study of diabetic kidney donors do not seem to indicate that there is an accelerated development of diabetic kidney disease (3–6). In fact, graft loss from DM in recipients is distinctly rare and donors who developed diabetes were, at least in the short term, not at a heightened risk for rapid progression of diabetic kidney disease (7–9). Moreover, case series of patients with a native single kidney and DM do not seem to suggest a higher risk of diabetic nephropathy (10,11).
Herein, we determined the incidence of DM after donation and addressed its association with important renal outcomes including GFR decay, proteinuria, hypertension, and also end-stage renal disease (ESRD).
Methods
Study population
Detailed account of the University of Minnesota donor program has been published previously (12, 13). Exclusion criteria for donation include any proteinuria (urinary protein >150 mg/day or urinary albumin/ creatinine >30 mg/g), body mass index (BMI) > 30 kg/m2 unless physical examination results warranted acceptance; and diabetes. The University of Minnesota transplant program policy does not exclude donors with family history of type 2 DM (T2DM). We counsel the offspring of a potential recipient with T2DM about their lifetime risk for the condition and have generally accepted such donors if their weight is normal. Potential donors who have multiple siblings with T2DM in addition to a parent with DM or more than one immediate family member with diabetic kidney disease, however, are strongly discouraged from donating, particularly if they are African-American or Hispanic. Prior to 2002, all potential donors wishing to donate to a family member with T2DM required a normal glucose tolerance test (GTT). After 2002, only those with more than one immediate family member with T2DM, women with history of gestational DM and those with fasting blood glucose 99 to 110 mg/dL underwent GTT. Hypertension has historically been a contraindication to donation. More recently, we have accepted Caucasian donors > 55 years of age and non-Caucasian donors > 60 years of age whose hypertension is well-controlled with a single drug and without evidence of end-organ damage including absence of hypertensive retinal changes as documented by an ophthalmologist.
At regular intervals, kidney donors are sent a survey regarding their health. Donors are also asked to provide laboratory test results and copies of records (or, if not done, to have these tests). Also, with donors’ consent, we contact their local clinics for recent history, physical examination notes, and laboratory test results, including serum creatinine, glucose, urinalysis, and urinary protein measurement. This study was approved by the University of Minnesota Institutional Review Board (HSC #0301M39762) and was supported by the National Institutes of Health (5P01 DK013083).
Exposures and outcomes
DM was determined from donors’ self-report of treatment with diet, oral agents or insulin. Donors provided the name and start date for antidiabetic medications and fasting blood sugar measurements and also HbA1c, when available.
To address the renal implications of DM after donation, we studied the association of DM with incident hypertension, proteinuria, rate of eGFR change prior to and after DM development, and ESRD. We defined proteinuria by a urinary albumin excretion rate > 30 mg/g creatinine, 24 hour urinary protein >200mg/day or ≥ 2+ on urine dipstick tests. ESRD was defined by needing dialysis, undergoing a kidney transplant, or being placed on the deceased donor waiting list for a transplant. To calculate the eGFR, we used the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (14). We previously showed that the CKD-EPI equation is more precise (than the MDRD Study equation) in living kidney donors who underwent GFR measurement with iohexol (15). Only creatinine values > 6 weeks post-donation were used because the goal was to model long-term trends in kidney function.
Statistical analysis
Continuous covariates were summarized as mean (standard deviation) and categorical characteristics were summarized as frequencies (percent). To test univariable differences between donors with and without DM, we used t-tests (continuous variables) and Chi-square or Fisher’s tests (categorical variables). Kaplan-Meier estimation was used to determine the cumulative incidence of diabetes, hypertension, and proteinuria over time since donation. Follow-up for these time-to-event outcomes was censored at the latest of the following: latest clinical encounter, latest receipt of health survey, or information collected at time of death.
To estimate the effect of incident DM on eGFR trajectory we used linear mixed effects models. Specifically, we fit a model with fixed effects for age at donation, gender, BMI at donation, donation year, fasting glucose, eGFR, donor type, DM as cause of ESRD in the recipient, smoking, race and time since donation. Interaction terms between time and both donor type (related vs. unrelated) and eGFR were also used. Considering the post-donation compensatory increase in GFR, we allowed the eGFR trajectory to change at 15 years post-donation for all donors. Additionally, we allowed the eGFR trajectory to change seven years prior to DM diagnosis to allow for the fact that DM may cause changes in kidney function prior to clinical diagnosis, as well as at time of DM diagnosis. This linear spline basis allows for sufficient flexibility in the post-donation trajectory while remaining more interpretable than other basis expansions. The knot points at 15 years (entire cohort) and 7 years before diagnosis and at DM diagnosis were chosen based on modeling the eGFR trajectory using restricted cubic splines using the rcspline macro (16). Additionally, we wanted to assess whether or not the eGFR trajectory once donors developed DM differed by whether or not the donor also had hypertension and proteinuria. Specifically, indicators for whether diabetic donors developed hypertension and proteinuria up to five years post-DM were included in an interaction term with time from diabetes diagnosis date. To account for correlation among repeated measurements for the same donor, we included a random subject-specific intercept and random slopes. Individual eGFR trajectories were estimated using the empirical Bayes (best linear unbiased predictors) of the random effects (17).
In a sensitivity analysis to compare eGFR trajectories post-donation, we performed matching as follows: each diabetic donor was matched to five donors who survived without DM until at least the time their respective case developed diabetes (18). A linear mixed model was again fit while adjusting for the same fixed patient characteristics that were used for the main trajectory analysis.
We also quantified the association between DM and ESRD, proteinuria, and hypertension, by treating DM onset as a time-dependent covariate in a proportional hazard regression model. Covariates included for this analysis were age, gender, systolic blood pressure, diastolic blood pressure, BMI, fasting glucose, year of donation, smoking, relationship to recipient, eGFR at donation and also race. Covariates were selected using backward variable selection with p<0.05 to stay. In each model, time-dependent DM and hypertension (for proteinuria and ESRD outcomes) were included in the final model regardless of significance.
For all data analyses we used SAS version 9.4 (SAS Institute, Inc., Cary, NC). Graphics were produced in R version 3.3.2. All statistical tests were two-sided tests with p < 0.05 indicating statistical significance.
Results
Of the 4,362 living kidney donors at our center, 4,014 (92.2%) completed a health survey with the latest follow-up occurring at an average 17.7 years (sd 12.1 years) post-donation; 58.2% were women and 78.9% were related to their recipient; mean age at donation was 39.5 (11.7) years (Table 1). At donation, the mean serum creatinine level was 0.9 (0.2) mg/dL; eGFR, 99.2 (25.4) ml/min/1.73m2; BMI, 25.8 (4.3) kg/m2. Respondents were more likely to be women and less likely to be smokers but were otherwise comparable to non-respondents. When comparing those who did (n=309) and did not (n=3705) develop DM post-donation, the DM donors had a significantly higher donation BMI, serum glucose, serum creatinine and eGFR. They were younger and more likely to be related to their recipient, smoke, and be male (Table 1). For the entire cohort, the mean change in BMI from donation to last follow-up was 1.97 (4.2) kg/m2. For diabetic donors it was 3.9 (5.8) and for non-diabetic donors it was 1.8 (4.0) kg/m2, p <0.001.
Table 1.
General characteristics of donors studied (n = 4,014), mean (SD) or %
| Variable | All Donors | DM Donors (n=309) |
Non-DM Donors (n=3705) |
p value (DM vs. non-DM) |
|---|---|---|---|---|
| Age, years | 39.5 (11.7) | 38.0 (11.2) | 39.7 (11.7) | 0.01 |
| Caucasian | 95.0% | 91.9% | 95.2% | 0.01 |
| Related to recipient | 78.9 | 90.3 | 78.0 | <0.01 |
| Female gender | 58.2 | 52.1 | 58.7 | 0.03 |
| Smoker | 30.1 | 41.2 | 29.2 | <0.01 |
| Body mass index, kg/m2 | 25.8 (4.3) | 27.6 (4.8) | 25.7 (4.2) | <0.01 |
| Systolic blood pressure, mm Hg | 119.9 (13.0) | 119.6 (13.3) | 119.9 (13.0) | 0.70 |
| Diastolic blood pressure, mm Hg | 73.4 (9.9) | 74.1 (10.0) | 73.3 (9.9) | 0.17 |
| Recipient kidney disease | ||||
| Glomerulonephritis | 28.8 | 31.7 | 28.6 | 0.35 |
| Type 2 diabetes | 4.3 | 3.2 | 4.4 | |
| Other | 66.9 | 65.1 | 67.0 | |
| Serum glucose, mg/dL | 93.3 (14.6) | 97.5 (19.1) | 93.0 (14.1) | <0.01 |
| Serum creatinine, mg/dL | 0.90 (0.16) | 0.94 (0.17) | 0.90 (0.16) | <0.01 |
| eGFR (CKD-EPI), ml/min/1.73 m2 | 99.2 (25.4) | 111.0 (31.0) | 98.2 (24.7) | <0.01 |
| At latest follow-up | ||||
| eGFR (CKD-EPI), ml/min/1.73 m2 | 64.5 (16.4) | 63.5 (19.6) | 64.6 (16.1) | 0.37 |
| HTN | 32.5 | 74.0 | 29.0 | <0.01 |
| Proteinuria | 7.2 | 25.7 | 5.6 | <0.01 |
| CVD | 12.6 | 31.8 | 11.0 | <0.01 |
| BMI, kg/m2 | 27.8 (5.5) | 31.6 (6.1) | 27.5 (5.3) | <0.01 |
CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration equation; eGFR = estimated glomerular filtration rate; SD = standard deviation
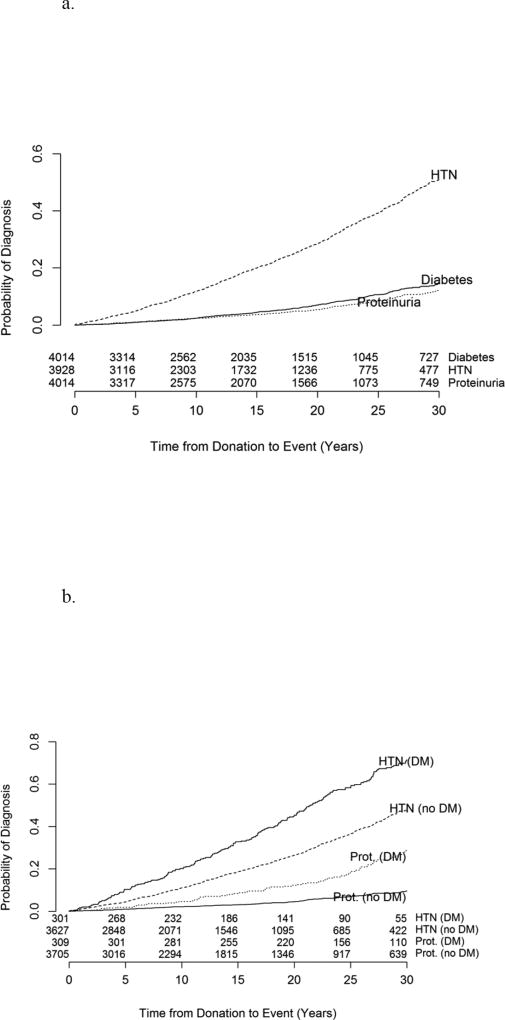
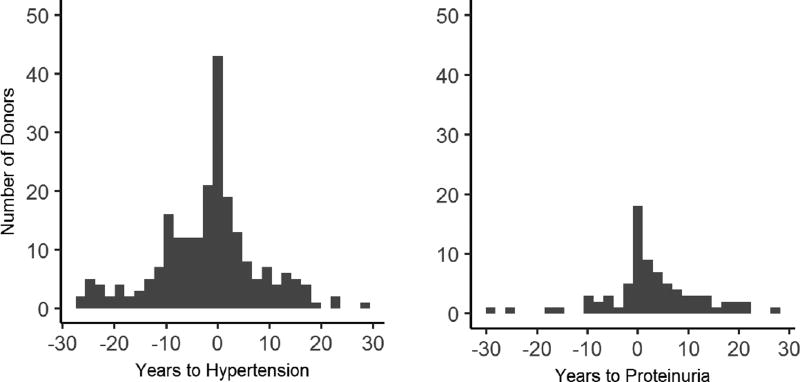
In total, 309 (7.7%) donors developed DM during the observed follow-up at a median age of 56.0 years and after a median of 18.0 years from donation. An average of 9.2 (range 0–34.7) years of follow-up was available post DM diagnosis. The cumulative incidence of diabetes, hypertension, and proteinuria in the entire cohort is shown in Figure 1a. The incidence of DM at 10, 20, and 30 years post-donation was 2.4%, 7.0%, and 14.5%, respectively. The incidence of proteinuria and hypertension was significantly higher for DM donors (log-rank p<0.0001) in comparison to non-DM donors (Figure 1b). Of the 309 donors who developed DM during the observed follow-up, 186 (60.2%) developed hypertension, 52 (16.8%) developed proteinuria, and 200 (64.7%) developed either hypertension or proteinuria either before or within the first five years post-diabetes diagnosis. To better dissect the temporal relationship between onset of DM and proteinuria, histograms depicting the time from diabetes diagnosis to diagnosis of proteinuria and HTN is shown in Figure 2. The majority of cases of proteinuria came after DM onset while HTN preceded DM in the majority. Of the 3705 non-diabetic donors, 952 (25.7%) developed hypertension, 199 (5.4%) developed proteinuria, and 1040 (28.1%) developed either condition at any point post-donation.
Figure 1.
(A) Cumulative incidence of diabetes, hypertension, and proteinuria in the entire cohort (n=4,014). (B) Cumulative incidence of hypertension and proteinuria in diabetic vs. non-diabetic donors. The incidence of proteinuria and hypertension was significantly higher for DM donors (log-rank p<0.0001) in comparison to non-DM donors.
Figure 2.
Temporal relationships between diabetes development and the development of proteinuria and hypertension (n=309). Time zero represents time of diabetes diagnosis.
eGFR Trajectory in Diabetic Donors and Matched Controls
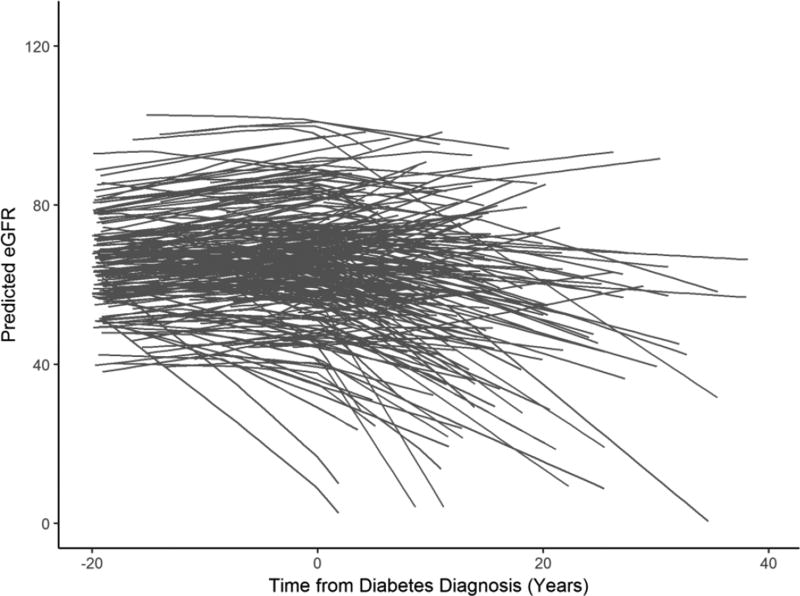
Of all donors, 264 diabetic donors and 2717 non-diabetic donors had at least one serum creatinine value >6 weeks post-donation and no missing covariate information and thus were used in the subsequent analyses. Donor-specific trajectories of predicted eGFR in relation to diabetes diagnosis date are shown in Figure 3. Out of the 264 DM donors, 191 had an increased predicted eGFR immediately before diabetes in comparison to donation and 73 decreased.
Figure 3.
Trajectory of predicted eGFR in diabetic donors prior to and after diabetes diagnosis. The data was restricted such that predicted values would correspond to dates between one year from donation and the year 2015.
In our first model which did not consider the proteinuria and hypertension status of donors, we found that after DM diagnosis, the difference in yearly change between diabetics and non-diabetics was −0.30 mL/min/year (95% CI (−0.55, −0.05); p = 0.02. We next looked at eGFR change after DM development accounting for the presence of hypertension and/or proteinuria up to five years post-diabetes; Table 2 gives the estimated coefficients from the linear mixed effect model. Here, the difference in the yearly eGFR change between diabetic and non-diabetic donors in the seven years prior to DM development was −0.08 (p=0.51). Following DM diagnosis, only the diabetic donors with both hypertension and proteinuria had a significantly steeper eGFR decline compared to a non-DM donor over the same time period: average yearly difference −1.10 mL/min/year for diabetic donors with HTN and proteinuria; p < 0.001; −0.19 mL/min/year for diabetic donors with hypertension but no proteinuria, p=0.29; −0.75 mL/min/year for diabetic donors with proteinuria but no hypertension, p=0.19; and −0.09 mL/min/year for diabetic donors without proteinuria or hypertension, p=0.63.
Table 2.
Model estimates of eGFR trajectory
| eGFR Estimate |
SE | p value | |
|---|---|---|---|
| a. Estimates of eGFR Trajectory and Trajectory Change | |||
| Non-diabetic donors related to recipient with donation eGFR of 105 mL/min/1.73m2 | |||
| First 15 years post-donation | 0.27 | 0.03 | <0.001 |
| > 15 years post-donation | −0.27 | 0.04 | <0.001 |
| Difference in eGFR change (diabetic vs. non-diabetic) | |||
| In the 7 years prior to DM diagnosis | −0.08 | 0.12 | 0.51 |
| After DM diagnosis | |||
| Hypertension, Proteinuria | −1.10 | 0.32 | <0.001 |
| Hypertension, No Proteinuria | −0.19 | 0.18 | 0.29 |
| No Hypertension, Proteinuria | −0.75 | 0.57 | 0.19 |
| No Hypertension, No Proteinuria | −0.09 | 0.20 | 0.63 |
| b. Model Parameter Estimates | |||
| Age at donation (per 10 years) | −4.55 | 0.21 | <0.001 |
| Female | 2.63 | 0.43 | <0.001 |
| BMI (per 1 unit) | −0.61 | 0.06 | <0.001 |
| Donation year (per decade) | 2.91 | 0.21 | <0.001 |
| Donation glucose (per 10mg/dL) | 0.08 | 0.15 | 0.61 |
| Donation eGFR (per 10 ml/min/1.73m2) (at time zero) | 2.39 | 0.12 | <0.001 |
| LRD (at time zero) | 0.56 | 0.61 | 0.36 |
| DM as cause of recipient ESRD | 0.11 | 0.44 | 0.80 |
| Smoking | 2.67 | 0.49 | <0.001 |
| White | −0.75 | 1.00 | 0.46 |
| Interaction of time from donation and LRD | −0.40 | 0.06 | <0.001 |
| Interaction of time from donation and donation eGFR | −0.00 | 0.00 | <0.001 |
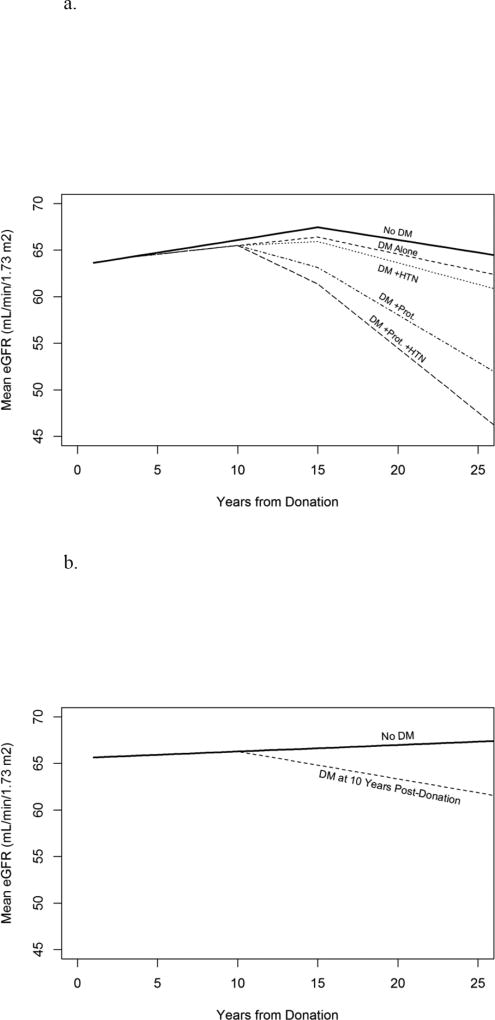
Figure 4a depicts the estimated mean eGFR trajectory for a representative donor who develops DM 10 years post-donation by whether or not the donor had proteinuria or hypertension (up to five years post-DM) as well as one who never develops DM. Estimates and standard errors for the data in this plot at five year intervals are presented in Table 3.
Figure 4.
(A) eGFR trajectory for a representative donor who does not develop diabetes (top line) and who does develop diabetes with stratification for proteinuria and hypertension (bottom 4 lines). A representative donor denotes the following characteristics: female, age 40 years, BMI 25 kg/m2, donation year 1985, glucose 100 mg/dL, eGFR 105 mL/min/1.73m2, non-smoker, no DM as cause of ESRD in recipient, LRD, and white race. (B) eGFR trajectory for a representative donor with and without diabetes diagnosis at 10 years post-donation.
Table 3.
Estimates (SE) at five year intervals for eGFR trajectory plot (Figure 4a) based on time from donation in five year intervals.
| Donor Type | 5 Years | 10 Years* | 15 Years | 20 Years |
|---|---|---|---|---|
| DM | ||||
| Hypertension, Proteinuria | 64.51 (0.51) | 65.46 (0.95) | 61.30 (1.85) | 54.43 (3.32) |
| HTN, No Proteinuria | 64.51 (0.51) | 65.46 (0.95) | 65.88 (1.20) | 63.58 (1.86) |
| No HTN, Proteinuria | 64.51 (0.51) | 65.46 (0.95) | 63.09 (2.98) | 58.01 (5.74) |
| No HTN, No Proteinuria | 64.51 (0.51) | 65.46 (0.95) | 66.35 (1.29) | 64.53 (2.05) |
| No DM | 64.68 (0.46) | 66.04 (0.46) | 67.40 (0.50) | 66.05 (0.48) |
time of diabetes diagnosis in trajectory plot
In the matched sensitivity analysis, annual eGFR change after DM development was 0.37 mL/min/year steeper in diabetic donors (95% CI −0.72, −0.01), p=0.045). Figure 4b depicts the change in eGFR for a representative donor with diabetes diagnosis at 10 years post-donation and without diabetes based on estimates from this analysis.
Consequences of Post Donation DM
In a multivariable proportional hazard analysis of all donors utilizing backwards selection and time-dependent covariates for DM, HTN and proteinuria (when appropriate for the outcome variable), post-donation DM was associated with an increased risk of post-donation hypertension (HR 2.19 (95% CI 1.74–2.75), p < 0.001) and proteinuria (HR 2.65 (95% CI 1.89–3.70), p < 0.001). DM was not associated, however, with an increased risk of ESRD (HR 0.52 (95% CI 0.21–1.26), p=0.15).
Discussion
These results indicate that the risk factors for the development of DM after donation are similar to what is seen in the general population including higher BMI and serum glucose levels. Donors who develop DM, similar to diabetics with 2 kidneys, are more likely to develop hypertension and proteinuria and their eGFR change exceeds that of non-diabetic donors only if they have hypertension and proteinuria which is also analogous to what is seen in the general population.
In total, 7.7% of donors developed DM at a median age of 56 years and thus would have to contend with diabetes and its related complications for 20 or more years. Although DM accounts for approximately half of all ESRD cases in the general population, only 12% to 22% of ESRD cases in kidney donors have been attributed to DM (13, 19–23). This lower incidence of ESRD from DM in kidney donors may reflect the fact that many people who are at very high risk of DM are generally excluded from donation. In addition, kidney donors tend to be healthier. Moreover, most of ESRD from diabetes occurs in ethnic minorities which are not sufficiently represented in this analysis. The link between obesity and reduced GFR, proteinuria, and ESRD in kidney donors is strong (13, 24). Most recently, Locke et al. reported that obese donors are almost twice more likely to develop ESRD in the first 20 years after donation (24). On the other hand, very little data exists regarding the fate of kidney donors who had elevated serum glucose at donation. To address this, we recently compared the outcomes of donors with fasting pre-donation glucose <100, 100–109, 110–125, and >126 mg/dL (25). These cutpoints represent what was considered normal fasting glucose in different eras according to the American Diabetes Association. Compared to donors with fasting blood sugar (FBS) < 109 mg/dL, donors in the 2 higher categories were more likely to develop hypertension and diabetes. Donors with FBS >126 mg/dL were more likely to become proteinuric, HR 1.83 (95% CI 1.01, 3.32). There were no differences in the risk of reduced eGFR amongst these groups. Lastly, many donate to a family member with GN. Due to its familial nature and the fact that GN has a more aggressive course than DM, this may account for the lower percentage of ESRD caused by DM in the donor population compared to the general population. In fact, we have recently shown that most of ESRD in the first few years after donation were due to FSGS (23).
These data do not support the generally prevailing conviction that hyperfiltration seen in many diabetic individuals will be additive to the hyperfiltration driven by uninephrectomy to cause adverse renal consequences. The prevalence of proteinuria in diabetic donors is not different than what is reported in diabetic individuals with two kidneys nor is their prevalence of hypertension higher (26). Moreover, the rate of GFR change in diabetic donors is similar to two-kidney hypertensive microalbuminuric diabetics such as the participants of the Irbesartan Diabetic Nephropathy Trial (27). In that trial, participants receiving placebo experienced a 1.2 ml/min/1.73m2 per year GFR decline. Clearly, not having actual two-kidney diabetic controls in this current analysis warrants extreme caution regarding conclusions about one kidney versus two kidney diabetes course. It is possible that since hyperfiltration is nearly complete in the first few years after donation, no additive hyperfiltration is taking place by the time diabetes develops. Moreover, hyperfiltration in kidney donors is mostly due to an increase in the glomerular surface area rather than augmentation in the intraglomerular pressure such as that seen with diabetes (28).
An average of 9.2 (range 0–34.7) years of follow-up was available post DM diagnosis. Data from the United Kingdom Prospective DM Study (UKPDS) indicate that for an individual with normoalbuminuria at onset of DM, it will take a median of 19 years before they advance to each subsequent stage of nephropathy (microalbuminuria, macroalbuminuria and, finally, elevated creatinine or ESRD); for those with microalbuminuria at DM onset it was a median of 11 years (29). Thus for a new diabetic donor with normoalbuminuria, ESRD is unlikely to occur during the lifetime of a donor. If microalbuminuria is, however, present, ESRD would develop at a median of 22 years which would put the diabetic donor at roughly age 80. In our series, 40% of diabetic donors received an ACE inhibitor or angiotensin II receptor blocker. These agents may account for some of the observed benign nature of their kidney function. How much impact these agents had on reduction of the degree of proteinuria and level of kidney function cannot be ascertained without performing the measurement while off these agents which was not done here.
There are limitations to our findings: DM and hypertension were self-reported and only verified in those who provided laboratory testing, a list of their medications and records from their physicians; 72% of all donors. The concordance of self-reported drug treated DM and hypertension with medical record abstraction is excellent (30, 31). ESRD in living donors is a rare event and thus we are unable to detect a difference in incidence of ESRD related to post-donation DM onset. We also cannot be certain that the proteinuria observed in diabetic donors is indeed caused by DM as we don’t have histological renal confirmation or detailed retinal exam. Lastly, the majority of the donors studied were white but 75% of US donors are white (32).
Collectively, these data suggest that current routine practices in Caucasian donors that exclude prediabetics and those with strong family history of diabetic kidney disease need to be continued. The development of diabetes results in a rate of GFR decline that is faster than non-diabetic donors only if hypertension and proteinuria are present.
Acknowledgments
Authors Hassan N. Ibrahim, Scott Jackson, and David M. Vock had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Statistical analysis completed by authors SJ and DMV.
Funding for this research was provided by the National Institutes of Health (NIH) as part of the Program Project Grant Studies of Organ Transplantation in Animals and Men (5P01 DK013083).
The authors would like to thank Robert Bailey, Kathryn Long, Jody Rincon, and the numerous other staff from Information Services for Research and Reporting, Fairview and Transplant Research Organization, University of Minnesota for their dedicated follow-up of living donors.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- DM
diabetes mellitus
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- GTT
glucose tolerance test
- HR
hazard ratio
- T2DM
type 2 DM
Footnotes
Disclaimer
The NIH had no role in study design, collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241(1):F85–93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of Losartan on Renal and Cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 3.Gaston RS, Alveranga DY, Becker BN, Distant DA, Held PJ, Bragg-Gresham JL, Humar A, Ting A, Wynn JJ, Leichtman AB. Kidney and pancreas transplantation. Am. J. Transplant. 2003;3(Suppl. s4):64–77. doi: 10.1034/j.1600-6143.3.s4.7.x. [DOI] [PubMed] [Google Scholar]
- 4.Boucek P, Saudek F, Pokorna E, Vitko S, Adamec M, Koznarova R, Lanska V. Kidney transplantation in type 2 diabetic patients: A comparison with matched non-diabetic subjects. Nephrol. Dial. Transplant. 2002;17:1678–1683. doi: 10.1093/ndt/17.9.1678. [DOI] [PubMed] [Google Scholar]
- 5.Molnar MZ, Huang E, Hoshino J, et al. Association of pretransplant glycemic control with posttransplant outcomes in diabetic kidney transplant recipients. Diabetes Care. 2011;34(12):2536–41. doi: 10.2337/dc11-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim HN, Kukla A, Cordner G, Bailey R, Gillingham K, Matas AJ. Diabetes after kidney donation. Am J Transplant. 2010;10(2):331–7. doi: 10.1111/j.1600-6143.2009.02944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronson JW, Gillingham KJ, Sutherland DE, Matas AJ. Renal transplantation for type II diabetic patients compared with type I diabetic patients and patients over 50 years old: a single center experience. Clin Transplant. 2000;14(3):226–34. doi: 10.1034/j.1399-0012.2000.140308.x. [DOI] [PubMed] [Google Scholar]
- 8.Snyder J, Matas A, Leppke S, Israni A, Kasiske B. Causes of kidney allograft loss and death in the United States, 2000–2010 [abstract] [Accessed January 26, 2016];Am J Transplant. 2013 13(suppl 5) http://www.atcmeetingabstracts.com/abstract/causes-of-kidney-allograft-loss-and-death-in-the-united-states-2000-2010/ [Google Scholar]
- 9.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9(3):527–35. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 10.Sampson MJ, Drury PL. Development of nephropathy in diabetic patients with a single kidney. Diabet Med. 1990;7:258–260. doi: 10.1111/j.1464-5491.1990.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 11.Silveiro SP, DaCosta LA, Beck MO, Gross JL. Urinary albumin excretion rate and glomerular filtration rate in single kidney type 2 diabetic patients. Diabetes Care. 1998;21:1521–1524. doi: 10.2337/diacare.21.9.1521. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360(5):459–69. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim HN, Foley RN, Reule SA, et al. Renal function profile in white kidney donors: The first 4 decades. J Am Soc Nephrology. doi: 10.1681/ASN.2015091018. Epub ahead of print February 17, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Issa N, Kukla A, Jackson S, et al. Comparison of cystatin C and creatinine-based equations for GFR estimation after living kidney donation. Transplantation. 2014;98(8):871–7. doi: 10.1097/TP.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 16.Harrell Frank. SAS Macros for Assisting with Survival and Risk Analysis, and Some SAS Procedures Useful for Multivariable Modeling. http://biostat.mc.vanderbilt.edu/wiki/Main/SasMacros.
- 17.Weiss Robert. Modeling Longitudinal Data. New York: Spring-Verlag; 2005. [Google Scholar]
- 18.Wang Zhiwei. Optimized 1:N Case-Control Match Using SAS ® SAS Global Forum 2012. http://support.sas.com/resources/papers/proceedings12/088-2012.pdf.
- 19.United States Renal Data System. 2015 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2015. [Google Scholar]
- 20.Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86:162–7. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 21.Potluri V, Harhay MN, Wilson FP, Bloom RD, Reese PP. Kidney transplant outcomes for prior living organ donors. J Am Soc Nephrol. 2015;26:1188–94. doi: 10.1681/ASN.2014030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kido R, Shibagaki Y, Iwadoh K, et al. How do living kidney donors develop end-stage renal disease? Am J Transplant. 2009;9(11):2514–9. doi: 10.1111/j.1600-6143.2009.02795.x. [DOI] [PubMed] [Google Scholar]
- 23.Matas AJ, Hays RE, Ibrahim HN. A case-based analysis of whether living related donors listed for transplant share ESRD causes with their recipients. Clin J Am Soc Nephrol. 2017;12(4):663–68. doi: 10.2215/CJN.11421116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locke JE, Reed RD, Massie A, et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. 2017;91(3):699–703. doi: 10.1016/j.kint.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley RN, Issa N, Berglund D, et al. The case against declining donors with impaired fasting glucose. J Am Soc Nephrology. 2015;26 (Abstract edition): 868A. [Google Scholar]
- 26.Ibrahim HN, Hostetter TH. Diabetic nephropathy. J Am Soc Nephrol. 1997;8(3):487–93. doi: 10.1681/ASN.V83487. [DOI] [PubMed] [Google Scholar]
- 27.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 28.Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC. Longitudinal study of living donor glomerular dynamics after nephrectomy. J Clin Invest. 2015;125(3):1311–8. doi: 10.1172/JCI78885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–32. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 30.Tisnado DM, Adams JL, Liu H, et al. What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care. 2006;44(2):132–40. doi: 10.1097/01.mlr.0000196952.15921.bf. [DOI] [PubMed] [Google Scholar]
- 31.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE. Concordance between respondent self-reports and medical records for chronic conditions: experience from the Veterans Health Study. J Ambul Care Manage. 2005;28(2):102–10. doi: 10.1097/00004479-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR Annual Data Report 2014: Kidney. Am J Transplant. 2016;(Suppl 2):11–46. [Google Scholar]