Table 2.
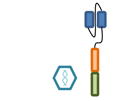
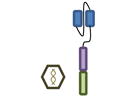
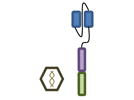
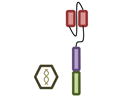
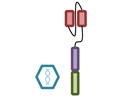
Design of BCMA targeted CAR T cell vectors and clinical trials for MM
| Institution | NCI | Bluebird Multi-Inst | Nanjing Legend | UPenn | MSK |
|---|---|---|---|---|---|
|
| |||||
| Overview |
|
|
|
|
|
|
| |||||
| scFv derived from | Murine Hybridoma | Murine Hybridoma | Murine Hybridoma | Human Library | Human Library |
|
| |||||
| Co-stimulatory domain | CD28 | 4-1BB | 4-1BB | 4-1BB | 4-1BB |
|
| |||||
| Gene transfer | Retrovirus | Lentivirus | Lentivirus | Lentivirus | Retrovirus |
|
| |||||
| Conditioning | Cyclophosphamide+ Fludarabine | Cyclophosphamide+ Fludarabine | Cyclophosphamide | None (cohort 1)> Cyclophosphamide | Cyclophosphamide+/-Fludarabine |
|
| |||||
| BCMA Ag required | >50% | >50% | “clear expression” | no requirement | >1% |
|
| |||||
| Clinicaltrials.gov Identifier | NCT02215967 | NCT02658929 | NCT03090659 | NCT02546167 | NCT03070327 |
|
| |||||
| Median prior lines | 7 | 7 | 3 | 9 | Not yet reported |
|
| |||||
| Efficacy | CR: 1 | CR: 4 | CR: 15 | CR: 1 | Not yet reported |
| VGPR: 4 | VGPR: 7 | VGPR: 13 | VGPR: 2 | ||
| PR: 3 | PR: 4 | PR: 7 | PR: 1 | ||
| 16 evaluable pts | 18 evaluable pts | 35 evaluable pts | 9 evaluable pts | ||
|
| |||||
| Legend |
|
||||
NCI National Cancer Institute, UPenn University of Pennsylvania, MSK Memorial Sloan Kettering, scFv single chain variable fragment, CD3 ζ T cell receptor zeta-chain
