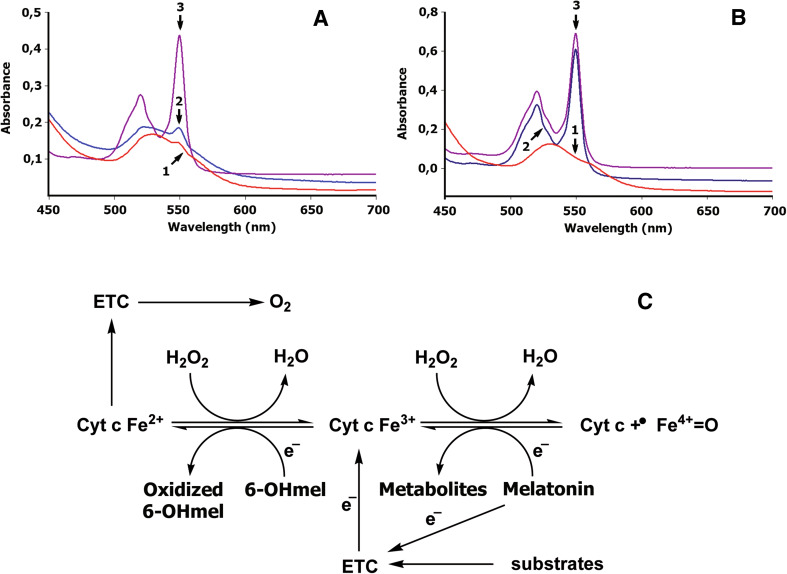
Fig. 6.
Melatonin and 6-hydroxymelatonin as regulators of bioenergetics of mitochondria in physiologic or pathological conditions. Reduction of oxidized cytochrome c by melatonin (a) and 6-hydroxymelatonin (b). Melatonin (a) or 6-hydroxymelatonin (b) was added to 0.025 mM of an oxidized cyt c (trace 1) dissolved in 10 mM Tris–HCl buffer (pH 7.4), to a final concentrations of 0.5 mM (trace 2); sodium dithionite was added to produce total reduction of cyt c (trace 3). The absorbance spectrum was recorded 30 min after addition of melatonin or 6-hydroxymelatonin as described previously [62]. Trace 1 oxidized cytochrome c, trace 2 cytochrome c reduced by melatonin (a) or by 6-hydroxymelatonin (b); trace 3 total reduction of cytochrome c incubated with melatonin (a) or 6-hydroxymelatonin (b) by sodium dithionite. c Melatonin could donate an electron to the Complex I of the ETC in physiologic conditions [90]. Accumulation of oxoferryl cytochrome c (cyt c + ·FeIV = O) induced by high levels of H2O2 could impair the cytochrome c-mediated electron shuttle between complex III and complex IV. Interaction of melatonin with oxoferryl hemoprotein restores the normal redox cycle of cytochrome c, protecting mitochondrial energy homeostasis under oxidative stress. Melatonin metabolite, 6-hydroxymelatonin, effectively reduces oxidized cytochrome c (cyt c Fe3+), thereby supporting electron flux through the respiratory chain, even when cytochrome c is intensively oxidized by high levels of H2O2. Thus, melatonin and 6-hydroxymelatonin interactions with both oxoferryl and ferricytochrome c could play a significant role in bioenergetics of mitochondria in physiologic or pathological conditions