Scheme 1.
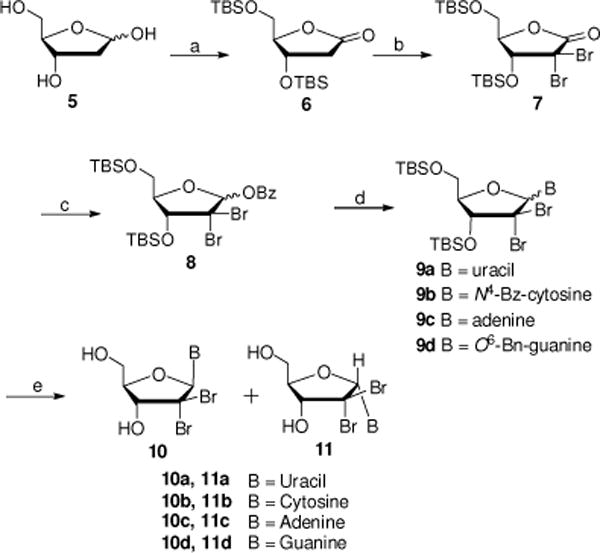
Reagents and conditions: (a) (i) Br2, H2O, rt, 5 d; (ii) TBDMCl, imidazole, DMF, rt, 24 h, 70% over two steps; (b) NBS, LiHMDS, THF, −78 °C to −10 °C, 4–6 h, 80%; (c) (i) DIBAL-H, toluene, −78 °C, 2 h, rt; (ii) BzCl, Et3N, DCM, 0 °C to rt, 12 h, 81%; (d) (i) nucleobase (Uracil, N4-Bz-cytosine, N6-diBoc-adenine or N4-diBoc-O6-Bn-Guanine), BSA, CH3CN, 60 °C, 30 min; (ii) TMSOTf, CH3CN, MW, 120 °C, 10 min; (e) for 9a and 9c: TBAF, THF, 0 °C, 1 h, 40–50% yield; for 9b: (i) NH3/MeOH, rt, 16–24 h; (ii) TBAF, THF, 0 °C, 1 h, 33% yield; for 9d: (i) TBAF, THF, 0 °C, 1 h; (ii) TFA, rt, 48–72 h, 33% yield.
