Figure 9.
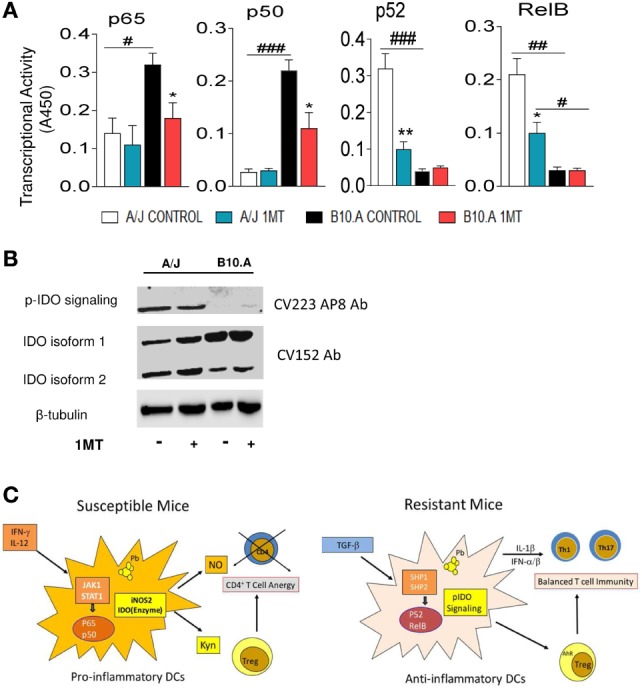
Phosphorylated indoleamine-2,3 dioxygenase (pIDO) of A/J mice activate the non-canonical pathway of NF-κB, whereas in B10.A dendritic cells (DCs) non-phosphorylated IDO use the canonical pathway of NF-κB activation. (A,B) Quantitation of p65, p50, p52, and RelB by enzyme-linked immunosorbent assay (ELISA) in nuclear extracts of DCs. Results are presented as absorbance at 450 nm (A450). Values are the mean ± SE of three independent experiments. The asterisks represent statistically significant differences between treatments (*p < 0.05, **p < 0.01, ***p < 0.001). The hash marks represent statistically significant differences between strains (#p < 0.05, ##p < 0.01, ###p < 0.001). (C) Expression of IDO and phosphorylated IDO was assessed by Western blot in lung homogenates of 1MT treated and untreated B10.A and A/J mice i.t. infected with 1 × 106 Paracoccidiodes brasiliensis yeasts. pIDO was revealed by a specific antibody that recognizes the phosphorylated ITIM moiety of IDO (CV223 AP8) and the enzyme IDO by a specific monoclonal antibody (CV152). (D) In susceptible B10.A mice, IDO is induced by IFN-γ that activates the canonical pathway of NF-κB and enhanced iNOS and IDO expression resulting in anergy of T cell immunity. In resistant A/J mice, TGF-β signaling induces the non-canonical pathway of NF-κB activation and continuous synthesis of TGF-β that confers an stable tolerogenic behavior to DCs that control immunity and tissue pathology.
