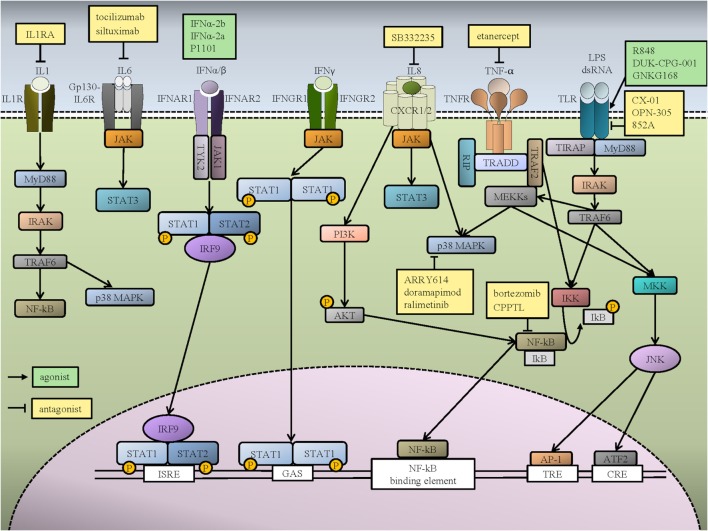
Figure 1.
Inflammatory signaling pathways in hematopoietic cells and potential therapeutic targets for myeloid malignancies. Interleukin (IL)-1β activates the IL-1 receptor (IL-1R), which causes dimerization and intracellular downstream signaling via MYD88 and IRAK. This activates multiple downstream pathways, including NF-κB and p38 MAPK. Two interleukin 6 (IL-6) molecules form a hexamer with two IL-6 receptors (IL-6R) and two GP-130 molecules, which signal via the JAK1–STAT3 pathway. The binding of IFN-α/β to IFNAR receptors activates TYK2 and JAK1, which phosphorylate STAT1 and STAT2. The association of IRF9 and phosphorylated STAT1 and STAT2 activates transcription by binding to IFN-stimulated response elements (ISREs). IFN-γ binding to IFNGR receptors promotes STAT1 phosphorylation by JAK. The STAT1 homodimer translocates to the nucleus and activates IFN-γ-activated site (GAS) sequences. IL-8 binds to its receptor, either CXCR1 or CXCR2, which can activate various downstream signaling pathways, including PI3K/AKT, JAK/STAT, and MAPK. There is extensive crosstalk between tumor necrosis factor alpha (TNF-α) and Toll-like receptor (TLR) signaling pathways. TNF-α binds to its receptor TNFR and activates IKK via RIP and TRAF2 recruitment by TRADD. IKK activation promotes IKB phosphorylation and release of NF-κB, which can then translocate to the nucleus. TNF-α binding also activates p38 and MEKK. The activation of MEKK causes JNK to stimulate AP-1, which binds to TPA DNA-response elements (TRE) and ATF2, which binds to cAMP-responsive elements (CRE). Activation of TLR by infectious molecules initiates the signaling pathway through MyD88, which recruits IRAK to bind TRAF6 and activate NF-κB and JNK pathways. Representative pathways agonists (green boxes) and antagonists (yellow boxes) that are either in preclinical or clinical investigation are shown.