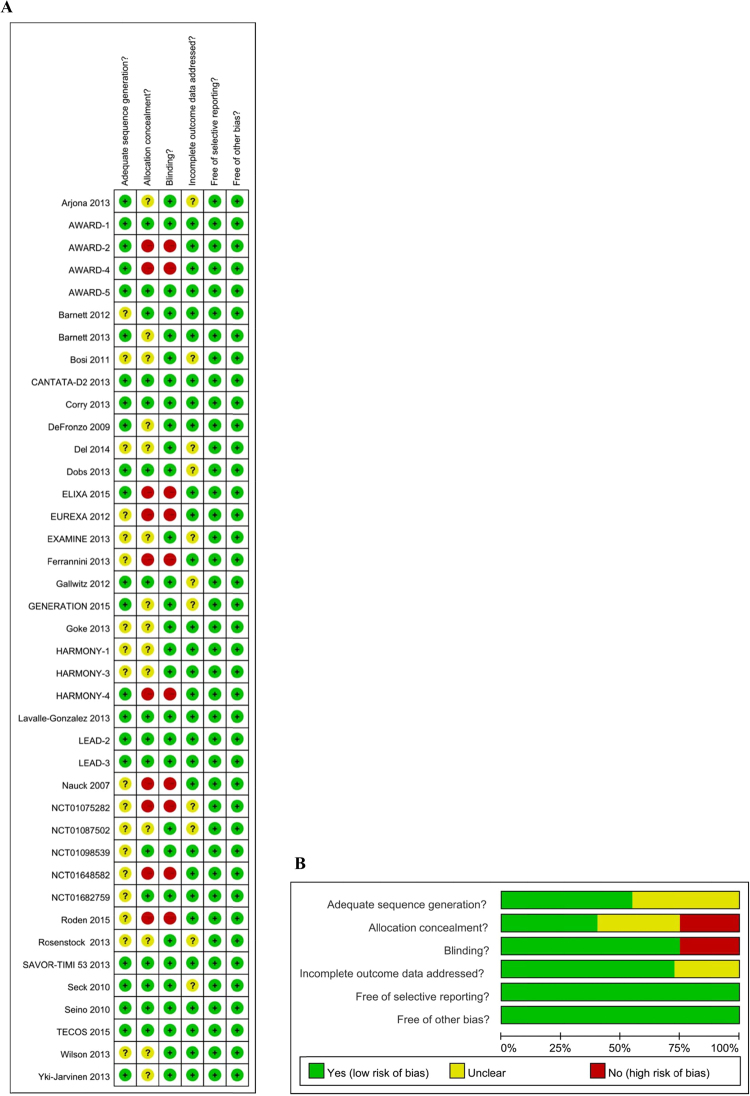
Figure 3.
Risk of bias assessment of included trials. The methodological quality assessment was performed by using the Cochrane Collaboration’s tool to assess risk of bias in for included trials. Allocation sequence generation was adequate in most of published trials except open-label trials and the unpublished trials which were judged as unclear. Open-label trials were judged as high risk in the allocation concealment and blinding. Eleven trials were judged as unclear risk of incomplete data, because these studies did not address the outcome analysis. Selective report biases were not identified in the included studies.