Abstract
In a case of 41-year-old man with mild nephropathy, Alport syndrome (AS) was diagnosed from the renal biopsy. However, the α5 chain of type IV collagen expressed in the glomerular basement membrane, which was the atypical staining pattern of AS. Genetic testing suggested autosomal recessive AS from heterozygous mutations at two positions in the type IV collagen α3 chain. These two gene mutations represented a new pattern of mutation and was suggested the association with an atypical α5 chain expression and mild phenotype.
Keywords: Alport syndrome, The type IV collagen α3 chain (COLA4A3)
Introduction
Alport syndrome (AS), first described in 1927 by Alport [1], is a hereditary progressive glomerular disease caused by genetic defects in the type IV collagen α chain [2]. AS is accompanied by bilateral perceptive deafness, lenticonus, and retinal lesions [3]. X-linked AS (XLAS) is caused by genetic anomalies in the type IV collagen α5 chain (COL4A5). In contrast, autosomal recessive AS (ARAS) occurs due to a homozygous or compound heterozygous mutations, and autosomal dominant AS occurs due to heterozygous mutations in the type IV collagen α3 chain (COL4A3) or type IV collagen α4 chain [4]. Microscopic hematuria is observed during initial stages, and proteinuria develops with disease progression. End-stage renal failure is reported to occur by the age of 40 years in 90% of male patients and 12% of female patients with XLAS [5]. ARAS is more severe and progresses more rapidly than XLAS; ARAS patients typically develop end-stage renal failure at a median age 21.8 years, regardless of gender [6].
Renal biopsy is fundamental for diagnosing AS, and type IV collagen staining provides important information regarding AS diagnosis [6]. Generally, the α3, α4, and α5 chains are not expressed in the glomerular basement membrane (GBM) in ARAS cases, although the α5 chain is expressed in the Bowman’s capsules, renal tubular basement membrane, and cutaneous basement membrane [7]. However, this typical staining pattern is not found in all ARAS cases, and although rare, atypical cases have been reported.
We report a case of ARAS with an atypical α chain expression pattern and mild renal impairment with gene mutations that have not been reported previously.
Case report
The patient was a 41-year-old man who was found to have occult hematuria when he was in elementary school. Renal biopsy at that time led to a diagnosis of minor glomerular abnormalities. He underwent no specific treatment. Thereafter, screening urinalysis at school was consistently positive for occult blood, but the patient remained untreated. He had not undergone urinalysis after becoming an adult. At 39 years of age, a health check-up revealed microscopic hematuria (5–10/high-power field of urine blood cells), urine protein of 3+, and urine protein level of 7.52 g/gCr. Thus, the patient was referred to our hospital. Blood tests revealed total protein of 5.5 g/dL, albumin of 3.3 g/dL, blood urea nitrogen of 19 mg/dL, and creatinine of 0.75 mg/dL, indicating hypoproteinemia with preserved renal function. Diabetes mellitus, collagen tissue disease, and infectious disease were ruled out.
The patient had no apparent family history of renal disease or other diseases, and there were no noteworthy physical findings. He had been aware of his hearing loss since the age of 37 years, but had not undergone detailed examination until visiting our hospital, where he was subjected to audiometry and diagnosed with bilateral sensorineural deafness. No ocular disorder was found.
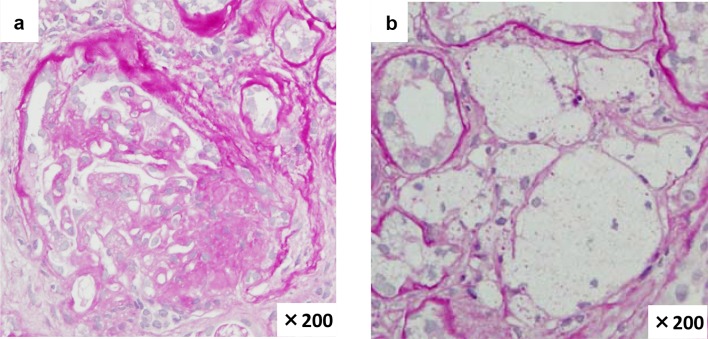
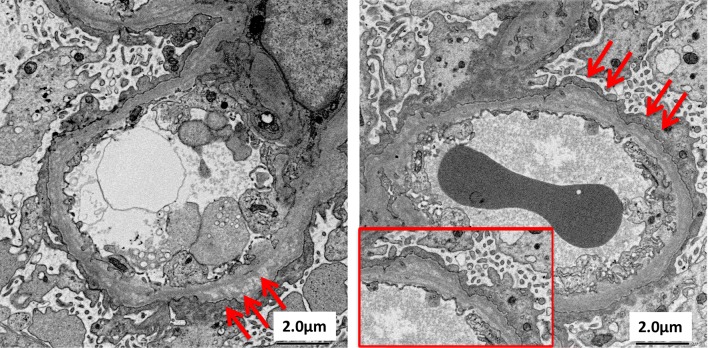
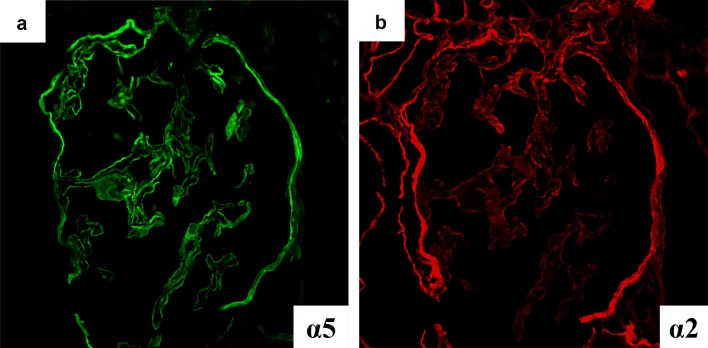
Based on the clinical course and results of the examination, we suspected chronic glomerulonephritis, and thus performed renal biopsy. The cortico-medullary ratio was 6:4. Approximately 13 glomeruli were included, and there was one sclerotic glomerulus. Increased mesangial matrix and segmental sclerosis were found in 4 glomeruli, accompanied by adhesion to the Bowman’s capsules. Periodic acid-methenamine silver staining revealed localized duplication of the GBM. Prominent aggregation of foam cells was observed in the interstitium (Fig. 1). Immunofluorescence staining yielded no specific findings. Electron microscopic findings included uneven thickening, splitting, and lamellation of the GBM (Fig. 2). Type IV collagen staining showed normal expression of the α5 chain and relatively weak but positive expression of the α2 chain in the GBM. Both the α2 and α5 chains showed normal expression in the Bowman’s capsules (Fig. 3).
Fig. 1.
Light microscopy findings. a Segmental glomerular sclerosis in one of thirteen glomeruli (PAS ×200). b Foam cells in the interstitium (PAS ×200)
Fig. 2.
Electron microscopy findings. Irregular distribution of thickening, lamellation and splitting in GBM (arrows)
Fig. 3.
Type IV collagen staining findings. a α5 Chain showed positive staining in the GBM and BC. b α2 Chain showed weak positive staining in the GBM and normally in BC
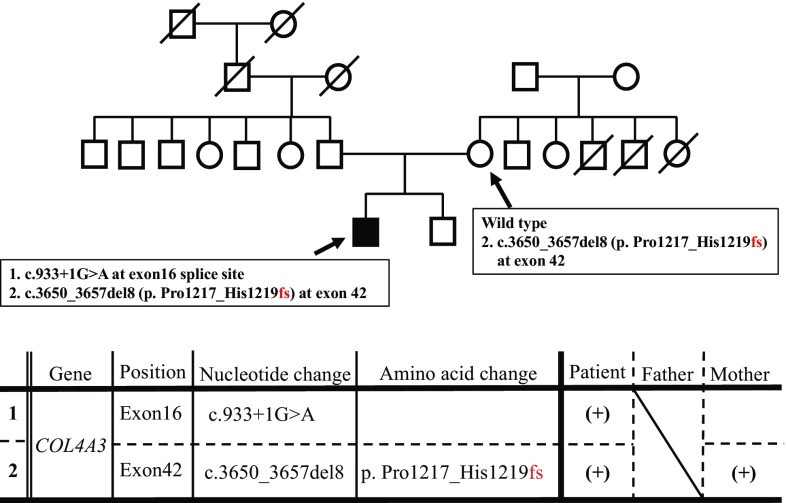
These findings were atypical of male XLAS or ARAS and failed to lead to a definitive diagnosis of AS. Therefore, gene analysis was conducted for the patient and his mother after they provided consent for testing. No sample was collected from the patient’s father because he did not provide consent for gene analysis. Genetic testing revealed two mutations: a splicing mutation in exon 16 of COL4A3 and a deletion mutation in exon 42 of COL4A3 (Fig. 4). There was no mutation in the COL4A4 gene of this patient. The patient’s mother also had a deletion mutation in exon 42, while the splicing mutation in exon 16 was considered as derived from the father possibly. Direct sequencing of cDNA confirmed that this splicing mutation showed exon skipping because of the c.933+1G>A mutation in exon 16. He was treated with angiotensin II receptor blocker as renoprotective therapy.
Fig. 4.
Mutational analysis. This patient had two mutations. 1 Splicing mutation, c.933+1G>A in COL4A3 exon16. 2 Frameshift mutation, c.3650_3657del8 (p. Pro1217_Gly1219 fs) in COL4A3 exon42. The frameshift mutation in COL4A3 exon42 was found in the gene of this patient’s mother, too. COL4A3 type IV collagen α3 chain
Discussion
We report a 41-year-old patient with abnormal urinalysis and mild renal impairment who was diagnosed with ARAS. Although AS was suspected from renal biopsy findings, atypical type IV collagen α chain staining was observed. Therefore, the diagnosis was established by genetic testing. This case represents AS with gene mutations that have not been reported previously.
Interestingly, this patient had only mild renal impairment at the age of 41 years despite ARAS. We suspected that the deviation from the typical type IV collagen (α2 and α5) staining pattern was associated with his preserved renal function. The α chain immunostaining patterns vary among the kidneys of healthy subjects and XLAS or ARAS patients. In this case, the GBM showed normal expression of the α5 chain and weak but positive expression of the α2, and both the α2 and α5 chains showed normal expression in the Bowman’s capsules; this pattern is atypical for ARAS (Table 1).
Table 1.
Comparison of immunofluorescence staining for α2 and α5 chain in the Bowman’s capsule and GBM
| GBM | Bowman’s capsule | |||
|---|---|---|---|---|
| α2 | α5 | α2 | α5 | |
| Control | Positive | Positive | Positive | Positive |
| XLAS | Positive | Negative | Positive | Negative |
| ARAS | Positive | Negative | Positive | Positive |
| This case | Positive | Positive | Positive | Positive |
ARAS autosomal recessive Alport syndrome, GBM glomerular basement membrane, XLAS X-linked Alport syndrome
Normal or partial expression of the α5 chain has been observed on rare occasions in patients diagnosed with ARAS [3, 8–10]. Storey et al. explained that the structure of type IV collagen can vary for different patterns of gene mutation [11]. Although type IV collagen in the GBM is not formed by nonsense mutations or other mutations that insert a termination codon in the downstream region, type IV collagen with an abnormal structure may be formed by missense mutations. Therefore, the results of immunostaining are not necessarily reliable in AS diagnosis, and the differences in collagen structure because of the type of gene mutation may be related to the rate of progression of impairment in renal function. Similar cases have been reported for XLAS; Hashimura et al. reported that XLAS patients with non-truncating mutation, in-frame deletion, or splice-site mutation expressed the α5 chain on GBM. They showed less urinary protein and developed into end-stage renal failure at a later age than those without α5 chain expression. α5 chain expression in XLAS patients appears to be related to milder clinical manifestations [12]. Jais et al. reported that there was a difference in the progression of renal impairment between missense mutations and nonsense mutations [13]. Storey et al. reported that nonsense mutations did not produce α3/α4/α5 heterotrimers, and α5 chain expression was absent in immunofluorescence staining. These findings were associated with early onset renal failure. While missense mutations produced an abnormal GBM that was less likely to be deleterious and expressed the α5 chain, these mutations were associated with late-onset renal failure [11]. Therefore, we considered that the presence or absence of the α5 chain which varied with the pattern of gene mutation was related to the prognosis of renal function, regardless of whether patients have XLAS and ARAS. However, no distinct association between genotype and phenotype has been observed with regards to any genotype of AS [14].
Our patient had two heterozygous mutations in COL4A3. The splicing mutation was a 45-bp deletion that skipped c.933+1G>A in exon 16, which is an in-frame mutation. We built up a hypothesis that the α3/α4/α5 heterotrimer was formed and an abnormal GBM was produced by the in-frame mutation, which was not likely to be deleterious. Therefore, this in-frame mutation might be associated with mild renal dysfunction at the age of 41 years. The other mutation in this patient was c.3650_3657del8 bp in exon 42, which was a frameshift causing truncation. Previously, Oka et al. reported that renal prognosis was not associated the presence of truncating mutations at a single allele [9]. Thus, this mutation does not appear to be related to renal dysfunction.
No previous studies have reported ARAS patients with normal renal function in their 40s. However, Oka et al. reported that a 28-year-old ARAS case of α5 staining positive for GBM had not progressed to chronic renal failure, which was similar to our case [9]. Thus, our patient, a male in his 40s with ARAS, was a rare case with mild renal dysfunction. It was possible that the splicing site mutation in exon 16 was de novo or the mutation in exon 42 was on the same allele, since we could not analyze the gene in the patient’s father and his sibling. To confirm the genetic diagnosis, we repeatedly recommended this patient’s family to receive genetic testing, but we were unable to obtain consent. We considered to sequence subcloned PCR product of cDNA of COL4A3 of this patient. But it was quite difficult to perform total sequencing because there are almost 3500 kbp between exon 16 and 42. Therefore, this patient’s family genotype was not completely revealed.
It was difficult to diagnose the patient with AS based on clinical and pathological findings alone, and genetic testing was utilized for diagnosis. However, genetic testing is not performed in all patients. The need for genetic testing should be determined according to the patient’s background, including family history, age, and renal prognosis. Considering these factors, genetic testing may be useful for obtaining a definitive diagnosis of AS.
Compliance with ethical standards
Conflict of interest
There is no conflict interest.
Research involving human participants informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Alport AC. Hereditary familial congenital haemorrhagic nephritis. Br Med J. 1927;1:504–506. doi: 10.1136/bmj.1.3454.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross O, Perin L, Deltas C. Alport syndrome from bench to bedside: the potential of current treatment beyond RAAS blockade and the horizon of future therapies. Nephrol Dial Transplant. 2014;29(suppl 4):iv124–iv130. doi: 10.1093/ndt/gfu028. [DOI] [PubMed] [Google Scholar]
- 3.Gubler MC, Knebelmann B, Beziau A, Broyer M, Pirson Y, Haddoum F, Kleppel MM, Antignac C. Autosomal recessive Alport syndrome: immunohistochemical study of type IV collagen chain distribution. Kidney Int. 1995;47:1142–1147. doi: 10.1038/ki.1995.163. [DOI] [PubMed] [Google Scholar]
- 4.Rana K, Tonna S, Wang YY, Sin L, Lin T, Shaw E, Mookerjee I, Savige J. Nine novel COL4A3 and COL4A4 mutations and polymorphisms identified in inherited membrane diseases. Pediatr Nephrol. 2007;22:652–657. doi: 10.1007/s00467-006-0393-y. [DOI] [PubMed] [Google Scholar]
- 5.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Dahan K, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC. X-linked Alport syndrome: natural history and genotype–phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol. 2003;14:2603–2610. doi: 10.1097/01.ASN.0000090034.71205.74. [DOI] [PubMed] [Google Scholar]
- 6.Heidet L, Gubler MC. The renal lesions of Alport syndrome. J Am Soc Nephrol. 2009;20:1210–1215. doi: 10.1681/ASN.2008090984. [DOI] [PubMed] [Google Scholar]
- 7.Kashtan CE, Michael AF. Alport syndrome. Kidney Int. 1996;50:1445–1463. doi: 10.1038/ki.1996.459. [DOI] [PubMed] [Google Scholar]
- 8.Heidet L, Arrondel C, Forestier L, Cohen-Solal L, Mollet G, Gutierrez B, Stavrou C, Gubler MC, Antignac C. Structure of the human type IV collagen gene COL4A3 and mutations in autosomal Alport syndrome. J Am Soc Nephrol. 2001;12:97–106. doi: 10.1681/ASN.V12197. [DOI] [PubMed] [Google Scholar]
- 9.Oka M, Nozu K, Kaito H, Fu XJ, Nakanishi K, Hashimura Y, Morisada N, Yan K, Matsuo M, Yoshikawa N, Vorechovsky I, Iijima K. Natural history of genetically proven autosomal recessive Alport syndrome. Pediatr Nephrol. 2014;29:1535–1544. doi: 10.1007/s00467-014-2797-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang F, Ding J, Zhang H, Zhao D, Yu L, Xiao H, Yao Y, Zhong X, Wang S. Genotype–phenotype correlations in 17 Chinese patients with autosomal recessive Alport syndrome. Am J Med Genet A. 2012;158:2188–2193. doi: 10.1002/ajmg.a.35528. [DOI] [PubMed] [Google Scholar]
- 11.Storey H, Savige J, Sivakumar V, Abbs S, Flinter FA. COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J Am Soc Nephrol. 2013;24:1945–1954. doi: 10.1681/ASN.2012100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimura Y, Nozu K, Kaito H, Nakanishi K, Fu XJ, Ohtsubo H, Hashimoto F, Oka M, Ninchoji T, Ishimori S, Morisada N, Matsunoshita N, Kamiyoshi N, Yoshikawa N, Iijima K. Milder clinical aspects of X-linked Alport syndrome in men positive for the collagen IV α5 chain. Kidney Int. 2014;85:1208–1213. doi: 10.1038/ki.2013.479. [DOI] [PubMed] [Google Scholar]
- 13.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Verellen C, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Krejcova S, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC. X-linked Alport syndrome: natural history in 195 families and genotype–phenotype correlations in males. J Am Soc Nephrol. 2000;11:649–657. doi: 10.1681/ASN.V114649. [DOI] [PubMed] [Google Scholar]
- 14.Uzak AS, Tokgoz B, Dundar M, Tekin M. A novel COL4A3 mutation causes autosomal-recessive Alport syndrome in a large Turkish family. Genet Test Mol Biomarkers. 2013;17:260–264. doi: 10.1089/gtmb.2012.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]