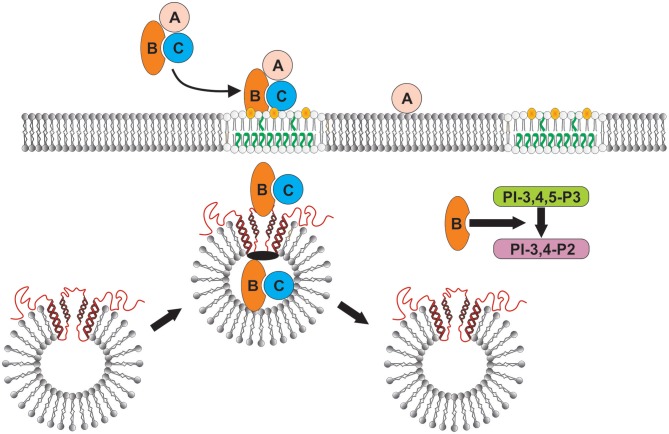
Figure 9.
Schematic model showing proposed CdtB-cellugyrin interaction. Cdt holotoxin binds to cells via cholesterol in the context of membrane lipid rafts. CdtB binding and internalization is further dependent upon its ability to interact with cholesterol. As a result of exposure to Cdt, cellugyrin (shown in red) containing SLMVs translocate from cytosol to membrane lipid rafts. We propose that this translocation leads to the association of CdtB with the cellugyrin-containing SLMVs. This interaction may involve direct binding to cellugyrin either on extra- or intra-vesicular loops or indirect association via an unidentified binding partner (shown in black). We further propose that CdtB is transported via SLMVs to intracellular target sites; for example sites containing PIP3 pools where the enzymatically active CdtB subunit is released from SLMVs and is then able to degrade the signaling lipid resulting in PI-3K blockade and toxicity.