Figure 5.
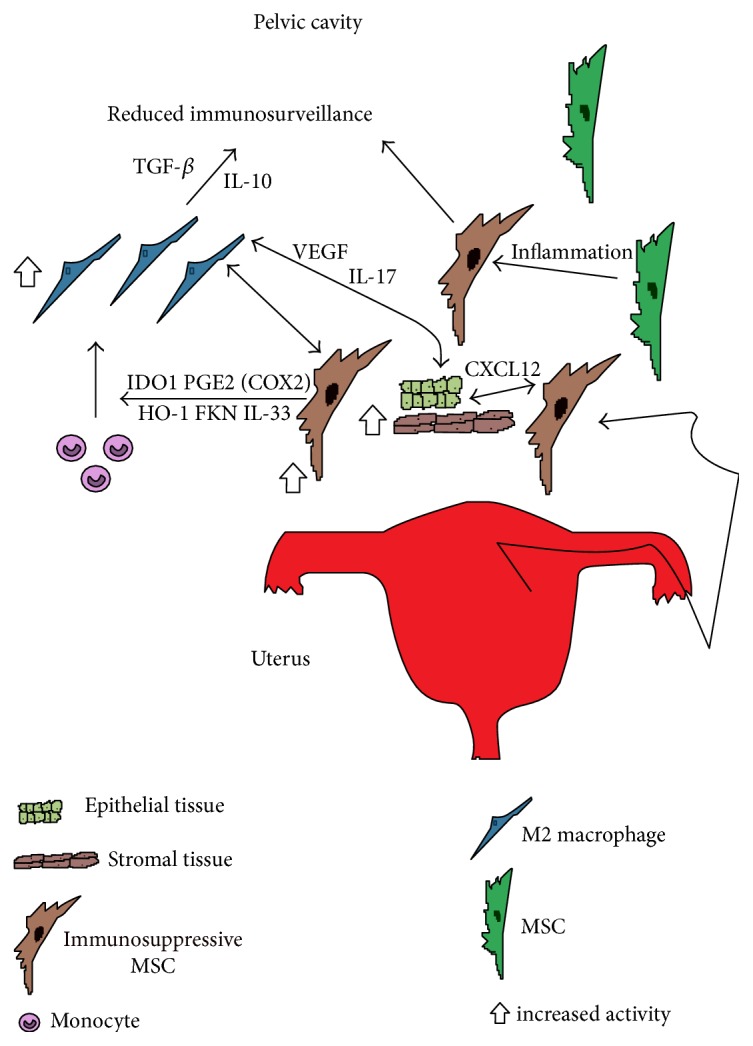
Schematic figure showing the role immunosuppressive ectopic MSC may be playing in the pathology of endometriosis. MSC along with stromal and epithelial endometrial tissue enter the pelvic cavity via retrograde menstruation. The highly inflammatory environment may induce MSC to become immunosuppressive to help promote tissue homeostasis. The recruited monocytes responding to the inflammatory environment and FKN, IL-33, IDO1, PGE2 via COX2, and HO-1 from immunosuppressive MSC may then polarize and differentiate into immunosuppressive M2 macrophages. Then, M2 macrophages may increase invasion of refluxed endometrial cells, repair ectopic lesions, and support their growth by inducing angiogenesis via their secretion of VEGF and IL-17 [58]. In addition, secretion of IL-10 and TGF-β by the M2 macrophages may suppress other recruited immune cells to reduce immunosurveillance in the pelvic cavity [58]. Also, ectopic MSC may directly support ectopic lesion growth by promotion of tissue repair, angiogenesis, migration, invasion, and suppression of apoptosis through CXCL12. The net effect may be large numbers of immunosuppressive MSC and M2 macrophages in the pelvic cavity that may directly support ectopic lesion growth, reduce immunosurveillance in the pelvic cavity, and reciprocally support each other's growth contributing to the development and progression of endometriosis. MSC: mesenchymal stromal cell; FKN: fractalkine; IDO1: indoleamine 2,3-dioxygenase 1; COX2: cyclooxygenase 2; HO-1: heme oxygenase 1; CXCL12: chemokine c-x-c motif chemokine ligand 12; VEGF: vascular endothelial growth factor; IL-17: interleukin 17; IL-10: interleukin 10; TGF-β: transforming growth factor-beta; IL-33: interleukin 33; PGE2: prostaglandin E2.
