Abstract
Background
Available data on the use of the Bioresorbable vascular scaffold (BVS) (Abbott Vascular, Santa Clara, CA) in real-world patients is limited, particularly in Asian populations. The aim of this study was to assess clinical outcomes of patients treated with a BVS in real-world practice in Taiwan.
Methods
This study focused on 156 patients with coronary artery disease and a total of 249 lesions who received BVS implantation from October 2012 to October 2015. The study’s primary endpoint was major adverse cardiac event (MACE), such as a myocardial infarction (MI), target vessel revascularization (TVR), target lesion revascularization (TLR), definite or possible scaffold thrombosis, cardiovascular death, and all-cause mortality during the thirty-day follow-up period. The secondary endpoint was MACE during the one-year follow-up period. Additionally, the composite clinical secondary endpoint was target lesion failure (TLF), which was called device-oriented composite endpoint.
Results
The average age of the patients was 60.34 ± 10.15 years, and 81.4% were male. The average of Syntax score was 12.42 ± 8.77 points. 44.2 % lesions were type B2 or C. At 31 days, one patient experienced a MACE (1/156) the composite of two TLF (2/249) with ST elevation MI, which was related to scaffold thrombosis. At one-year, 5.1 % (8/156) of the patients experienced a MACE and 3.6% (9/249) of the lesions experienced a TLF. There was no cardiovascular or all-cause mortality in the 30-day follow-up. The one-year cardiovascular and all-cause mortality rates were each 1.3%, respectively. Diabetes, ostial lesion, bifurcation lesion, and non-standard dual anti-platelet therapy (DAPT) were the strong associations of one-year TLF.
Conclusions
Even with difficult and complex lesions of patients in this study, acceptable outcomes were achieved with low definite or possible scaffold thrombosis rates after BVS implantation. And despite anatomical issues, it is important to complete standard DAPT.
Keywords: Bioresorbable vascular scaffold, Clinical outcomes, Single center experience
INTRODUCTION
Bioresorbable vascular scaffolds (BVSs) offer an emerging option in the percutaneous treatment of coronary artery disease (CAD). Their eventual resorption renders the artery free from a permanent metallic ‘cage’,1 and introduces the concept of a natural healing process following an initial percutaneous coronary intervention (PCI) without leaving any foreign materials in the body that could result in later adverse events.2 This allows for normal vasomotor vessel function to be restored, while also maintaining access for future coronary artery bypass grafting (CABG), if required. BVSs demonstrate favorable outcomes in patients with stable CAD with simple de novo coronary lesions.3,4 The results from several small studies and case reports suggest that BVSs can be utilized in a range of patients, including those with ST-segment elevation myocardial infarction (MI), long diffuse disease, complex bifurcation disease, severe calcified lesions, and in-stent restenosis lesions, demonstrating good short-term results.5-8 However, the role of BVSs in unselected real-world patients involving complex lesions remains inadequately evaluated, particularly in Asian populations.
The aim of this study was to assess the thirty-day and one-year clinical outcomes of BVSs in a real-world population treated at a single center in Taiwan.
MATERIALS AND METHODS
Patients and groups
From October 2012 to October 2015, 156 patients with CAD and a total of 249 lesions received BVS implantation in our hospital. The first 40 patients were enrolled in The ABSORB EXTEND study. Thirty-day and one-year clinical outcomes, such as target lesion revascularization (TLR), target vessel revascularization (TVR), MI, definite or possible scaffold thrombosis, cardiovascular mortality, and all-cause mortality were analyzed.
The Institutional Review Committee on Human Research at our institution approved the study protocol.
Definitions
MI definitions were in accordance with the most recent universal definition of MI.9 TVR was defined as a repeat percutaneous intervention (PCI) or CABG in a target vessel, and TLR was defined as a repeat PCI or CABG for a lesion in the previously treated segment or in an adjacent 5 mm segment. The occurrence of stent thrombosis was defined based on the Academic Research Consortium definition.10 Cardiovascular mortality was defined as death related to an MI and cardiac arrhythmia, and all-cause mortality was defined as death from any cause. Non-standard dual anti-platelet therapy (DAPT) was defined as patients who received only one anti-platelet therapy (aspirin, clopidogrel, or ticagrelor) and two anti-platelet therapy (clopidogrel plus cilostazol). Side branch injury was defined as when the flow became TIMI 0-1 flow after BVS implantation. Target lesion failure (TLF) was defined as the composite of cardiac death, target vessel-MI, or ischemic-driven TLR.
Study endpoints
The primary endpoint for this study was a major adverse cardiac event (MACE), such as an MI, TVR, TLR, definite or possible scaffold thrombosis, cardiovascular death, and all-cause mortality during the thirty-day follow-up period. The secondary endpoint was a MACE, such as an MI, TVR, TLR, definite or possible scaffold thrombosis, cardiovascular death, and all-cause mortality, during the one-year follow-up period. Composite clinical secondary endpoint was TLF which called device-oriented composite endpoint. Another composite clinical endpoint was MACE, which was designated the patient-oriented composite endpoint. The TLF (device-oriented composite endpoint) was chosen for the predictors of scaffold outcome analysis.
Statistical analysis
Data are expressed as mean ± standard deviation for continuous variables, or as counts and percentages for categorical variables. Continuous variables were compared using an independent sample t or Mann-Whitney U tests, and categorical variables were compared using a Chi-square statistic. Univariate and multivariate Cox regression analyses were performed to identify the one-year associations. Each correlation between the variables is expressed as a hazard ratio (HR) with 95% confidence interval (CI). All statistical analyses were performed using SPSS 22.0 (IBM. Corp., Armonk. NY, USA). A p value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics (Table 1)
Table 1. Baseline characteristics of study patients.
| Patients (N = 156) | |
| General demographics | |
| Age (year) | 60.34 ± 10.15 |
| Male gender (%) | 127 (81.4) |
| Clinical condition | |
| STEMI (%) | 2 (1.3) |
| NSTEMI (%) | 16 (10.3) |
| Unstable angina (%) | 58 (37.2) |
| Stable angina (%) | 80 (51.3) |
| Risk factors | |
| Hypertension (%) | 116 (74.4) |
| Diabetes (%) | 57 (36.5) |
| Current smoker (%) | 56 (35.9) |
| Prior myocardial infarction (%) | 16 (10.3) |
| Prior stroke (%) | 9 (5.8) |
| PAOD (%) | 1 (0.6) |
| Hyperlipidemia (%) | 106 (67.9) |
| Heart failure (%) | 5 (3.2) |
| Prior CABG (%) | 1 (0.6) |
| ESRD on maintenance hemodialysis (%) | 2 (1.3) |
| Laboratory examination | |
| Creatinine (mg/dL) (exclude ESRD) | 1.12 ± 1.13 |
| HbA1C (%) | 6.56 ± 1.21 |
| Total cholesterol (mg/dL) | 161.29 ± 38.32 |
| LDL-cholesterol (mg/dL) | 92.75 ± 32.57 |
| HDL-cholesterol (mg/dL) | 44.22 ± 11.47 |
| Syntax score | 12.42 ± 8.77 |
| F/U time (days) | 383.60 ± 292.35 |
| DAPT | |
| Aspirin + clopidogrel or ticagrelor | 136 (87.2) |
| Clopidogel + cilostazol | 8 (5.1) |
| Only aspirin or clopidogrel or ticagrelor | 12 (7.7) |
Data are expressed as mean ± SD or as number (percentage).
CABG, coronary artery bypass grafting; DAPT, dual anti-platelet therapy; ESRD, end stage renal disease; F/U, follow-up; HbA1C, glycohemoglobin; HDL, high density lipoprotein; LDL, low density lipoprotein; NSTEMI, non ST segment elevation myocardial infarction; PAOD, peripheral arterial occlusive disease; STEMI, ST segment elevation myocardial infarction.
The average age of study patients was 60.34 ± 10.15 years, and 81.4% were male. The most common clinical condition in the study patients was stable angina (51.3%). The other clinical conditions were unstable angina and non ST-segment elevation MI (NSTEMI) and rare ST elevation MI (STEMI, 1.3%) cases. Most of the patients had hypertension (74.4%) and hyperlipidemia (67.9%). The average SYNTAX score was 12.42 ± 8.77, and the average follow-up period was around 383.60 ± 292.35 days. Of all patients, 87.2% received standard DAPT, 5.1% received clopidogrel plus cilostazol, and another 7.7% received only one anti-platelet agent (aspirin or clopidogrel or ticagrelor). The DAPT was prescribed for at least 1 year following implantation of scaffolds.
Lesion and procedural characteristics (Table 2)
Table 2. Lesion and procedural characteristics.
| Lesions (N = 249) | |
| Patient number | 156 |
| Lesion number | 249 |
| Access (%) | |
| Radial | 249 (100) |
| Femoral | 0 (0) |
| Lesion-related artery (%) | |
| Left main artery | 4 (1.6) |
| Left anterior descending artery | 124 (49.8) |
| Left circumflex artery | 47 (18.9) |
| Right coronary artery | 74 (29.7) |
| Lesion type (%) | |
| Type B2 and C lesion | 110 (44.2) |
| Bifurcation lesion | 50 (11.1) |
| Left main | 6 (2.4) |
| Non left main | 44 (17.7) |
| Ostial lesion | 11 (4.4) |
| Left main lesion | 4 (1.6) |
| Chronic total occlusion | 14 (5.6) |
| In-stent restenosis lesion | 13 (5.2) |
| Pre-PCI angiography | |
| Pre-PCI stenosis (%) | 77.37 ± 8.52 |
| Pre-PCI MLD (mm) | 0.83 ± 3.22 |
| Pre-PCI RLD (mm) | 2.81 ± 0.51 |
| Pre-dilatation (%) | 155 (99.4) |
| Maximal pre-dilatation balloon diameter (mm) | 2.90 ± 0.44 |
| Maximal inflation (atm) | 17.00 ± 4.48 |
| Cutting balloon use | 9 (3.6) |
| Post-PCI angiography | |
| Post-PCI stenosis (%) | 12.18 ± 6.34 |
| Post-PCI MLD (mm) | 2.60 ± 0.45 |
| Post-PCI RLD (mm) | 2.97 ± 0.47 |
| Post-dilatation (%) | 153 (98.1) |
| Maximal post-dilatation balloon diameter (mm) | 3.14 ± 0.48 |
| Maximal inflation (atm) | 19.99 ± 5.52 |
| Scaffolds | |
| Diameter (mm) | 3.01 ± 0.37 |
| Length (mm) | 25.29 ± 4.53 |
| Intravascular imaging (%) | 209 (83.9) |
| Intravascular ultrasound | 196 (78.7) |
| Optical coherence tomography | 88 (39.3) |
| Rotational atherectomy (%) | 6 (2.4) |
| Complication of PCI (%) | 17 (6.8) |
| Branch vessel jailed | 10 (4.0) |
| Coronary perforation | 3 (1.2) |
| Coronary dissection | 3 (1.2) |
| Wire fracture | 1 (0.4) |
Data are expressed as mean ± SD or as number (percentage).
MLD, minimal luminal diameter; PCI, percutaneous coronary intervention; RLD, reference luminal diameter.
In this investigation, a total of 156 study patients had 249 lesions. All of the patients received PCI via a radial arterial approach. Approximately 44.2% of the patients had type B2 or type C lesions. There were 50 patients with bifurcation lesion, 6 patients had a left main (LM) bifurcation lesion, and 44 patients had non-LM bifurcation lesion. Furthermore, 4.4% of patients had ostial lesions, 1.6 % received LM stenting, 5.6% patients had chronic total occlusion (CTO) lesion, and 5.2% of patients received stenting for in-stent restenosis.
Pre-PCI reference luminal diameter (RLD) was 2.81 ± 0.51 mm, with the percentage of pre-PCI dilatation being 99.4%. The percentage of cutting balloon use was in 3.6%. The post-PCI RLD was 2.97 ± 0.47 mm. The percentage of post-PCI dilatation was 98.1% with the use of intravascular imaging (89.3%) including intravascular ultrasound (IVUS) (78.7%) and optical coherence tomography (OCT) (35.3%). The average diameter of scaffolds was 3.01 ± 0.37 mm, and the average length of scaffolds was 25.29 ± 4.53 mm. The complication rate of PCI was 6.8% (17 patients). Ten patients experienced a jailed branch vessel, 3 experienced a coronary perforation, 3 experienced a coronary dissection related to the scaffold deployment, and 1 experienced a wire fracture due to a severely calcified vessel.
Thirty-day and one-year clinical outcomes of the study patients and lesions (Table 3)
Table 3. Thirty-day and one-year clinical outcomes of study patients and lesions.
| Patients (N = 156) | |
| Lesions (N = 249) | |
| Thirty-day clinical outcomes | |
| MACE (%) | 1/156 (0.6) |
| MI (%) | 1/156 (0.6) |
| STEMI | 1/156 (0.6) |
| NSTEMI | 0 |
| Treated vessel MI | 1/156 (0.6) |
| Target-vessel revascularization (%) | 1/156 (0.6) |
| Target-lesion revascularization (%) | 2/249 (0.8) |
| Definite or possible scaffold thrombosis (%) | 1/156 (0.6) |
| Cardiovascular mortality (%) | 0 |
| All-cause mortality (%) | 0 |
| Target-lesion failure (%) | 2/249 (0.8) |
| One-year clinical outcomes | |
| MACE (%) | 8/156 (5.1) |
| MI (%) | 2/156 (1.3) |
| STEMI | 1/156 (0.6) |
| NSTEMI | 1/156 (0.6) |
| Treated vessel MI | 2/156 (1.3) |
| Target-vessel revascularization (%) | 8/156 (5.1) |
| Target-lesion revascularization (%) | 9/249 (3.6) |
| Definite or possible scaffold thrombosis (%) | 2/156 (1.3) |
| Cardiovascular mortality (%) | 2/156 (1.3) |
| All-cause mortality (%) | 2/156 (1.3) |
| Target-lesion failure (%) | 9/249 (3.6) |
MACE, major adverse cardiac event; MI, myocardial infarction; NSTEMI, non ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction.
Only one patient (0.6%) experienced a MACE (STEMI) during the thirty-day follow-up period. This case was a subacute definite scaffold thrombosis. During the one-year follow-up period, eight patients (5.1%) experienced a MACE. Of them, one patient experienced STEMI, another patient experienced NSTEMI and two patients had a treated vessel infarction. Overall, eight patients (5.1%) experienced a TVR and nine lesions (3.6%) experienced a TLR. Two patients (1.3%) experienced a definite scaffold thrombosis and cardiovascular death.
Univariate and multivariate Cox regression analyses about 1-year TLF (Table 4)
Table 4. Univariate and multivariate cox regression analyses about 1-year target lesion failure (TLF).
| Variables | Univariate analyses | Multivariate analyses | ||||
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| Risk factors | ||||||
| Diabetes | 5.332 | 1.108~25.672 | 0.037 | 8.558 | 1.223~59.881 | 0.031 |
| Dyslipidemia | 4.946 | 0.619~39.548 | 0.132 | |||
| Clinical condition | ||||||
| STEMI or NSTEMI | 0.043 | 0.001~825.819 | 0.532 | |||
| Angiographic factors | ||||||
| Type B2 or C lesion | 1.356 | 0.364~5.051 | 0.650 | |||
| Ostial lesion | 76.513 | 10.553~554.772 | < 0.001 | 13.202 | 1.668~104.490 | 0.014 |
| Bifurcation lesion | 5.415 | 1.453~20.175 | 0.012 | 11.227 | 2.029~62.132 | 0.006 |
| High Syntax score | 1.025 | 0.962~1.092 | 0.453 | |||
| In-stent restenosis | 2.887 | 0.361~23.096 | 0.318 | |||
| Peri-procedure MI | 0.911 | 0.189~4.384 | 0.907 | |||
| PCI complication | 2.6 | 0.325~20.794 | 0.368 | |||
| Medication | ||||||
| Non-standard DAPT | 7.953 | 2.133~29.658 | 0.002 | 15.914 | 2.813~90.029 | 0.002 |
CI, confidence interval; DAPT, dual antiplatelet therapy; MI, myocardial infarction; NSTEMI, non ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Univariate Cox regression analyses identified diabetes (HR: 5.332, 95% CI: 1.108-25.672, p = 0.037), ostial lesion (HR: 76.513, 95% CI: 10.553-554.772, p < 0.001), bifurcation lesion (HR: 5.415, 95% CI: 1.453-20.175, p = 0.012), and non-standard DAPT (HR: 7.953, 95% CI: 2.133-29.658, p = 0.002). Other variables such as dyslipidemia, MI, type B2 or C lesion, high Syntax score, peri-procedure MI, and PCI complications were not significantly different.
Multivariate Cox analysis revealed that diabetes (HR: 8.558, 95% CI: 1.223-59.881, p = 0.031), ostial lesion (HR: 13.202, 95% CI: 1.668-104.490, p = 0.014), bifurcation lesion (HR: 11.227, 95% CI: 2.029~62.132, p = 0.006), and non-standard DAPT (HR: 15.914, 95% CI: 2.813~90.029, p = 0.002) were independently associated with one-year TLF. Doing such an analysis on a small sample size may be very misleading indeed and prone to bias. This analysis should be heavily caveated, although it is reassuring to see no unsuspected predictors revealed from the data.
Case illustrations
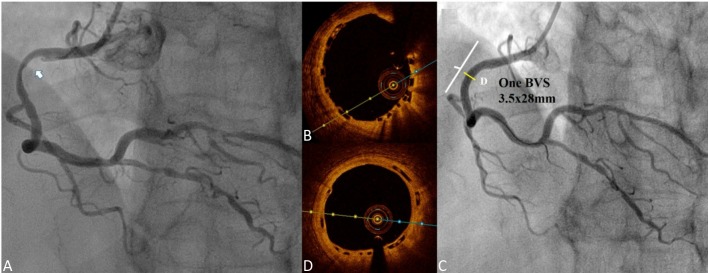
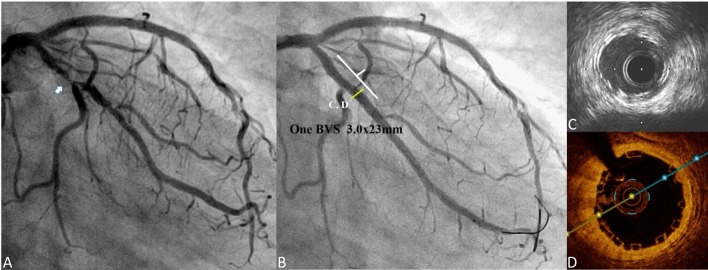
The first case involved a 51-year old man, who presented with angina, and received two 3.0 × 18 mm BVSs at the proximal to mid left anterior descending artery (LAD) for diffuse diseased lesions (Figure 1A). A 3.5-year follow-up coronary angiography (CAG) with OCT examination was performed. CAG of the left coronary artery showed fair coronary flow without stenosis (Figure 1B). OCT of LAD showed complete neointimal coverage of the residual BVS struts (black boxes) from proximal to mid LAD (Figures 1C-E). The overlap site showed two layers of BVS struts (Figure 1D). The second case was a 53-year-old man who presented with unstable angina, and received one 3.5 × 28 mm BVS for 70-80% stenosis at the proximal right coronary artery (RCA) (Figure 2A). After scaffold deployment, the flow of RCA did not have any limitation, and OCT examination showed good apposition of scaffold (Figure 2B). A 1-year follow-up CAG of RCA presented no restenosis (Figure 2C), and OCT also presented complete neointimal coverage of the residual BVS struts (Figure 2D). However, one new lesion with critical stenosis of left circumflex artery (LCX) was noted (Figure 3A). After one 3.0 × 23 mm BVS was deployed, good CAG result was noted (Figure 3B). IVUS showed double-layer struct, but could not evaluate the condition of BVS clearly (Figure 3C). OCT showed good apposition of BVS (Figure 3D).
Figure 1.
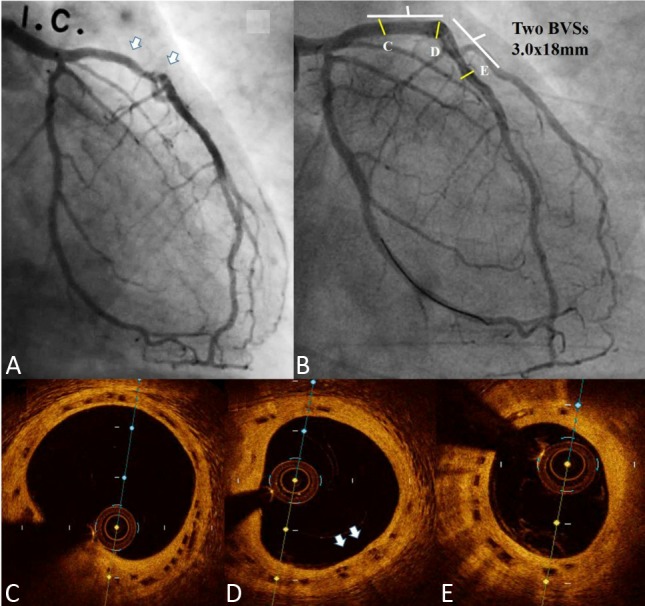
A: Coronary angiography (CAG) of the left coronary artery (LCA): Proximal to mid segment of left anterior descending artery (LAD) showed diffuse diseased lesions (white arrows); B: CAG of LCA: 3.5-year follow-up CAG showed fair coronary flow without stenosis; (C, D, and E) Optical coherence tomography (OCT) of LAD showed complete neointimal coverage of the residual bioresorbable vascular scaffold (BVS) struts (black boxes) from proximal to mid LAD (C-E). D: The overlap site showed two layers of BVS struts (white arrows).
Figure 2.
(A) Coronary angiography (CAG) of right coronary artery (RCA): 70~80% stenosis at proximal RCA (white arrow); (B) optical coherence tomography (OCT): good apposition of scaffold; (C) 1-year follow-up CAG of RCA presented no restenosis; (D) 1-year follow-up OCT of RCA: complete neointimal coverage of the residual bioresorbable vascular scaffold (BVS) struts.
Figure 3.
(A) Coronary angiography (CAG) of left coronary artery (LCA): one new lesion with critical stenosis of proximal left circumflex artery (LCX) was noted (white arrow); (B) CAG of LCA: fair coronary flow of LCX after bioresorbable vascular scaffold (BVS) deployment; (C) Intravascular ultrasound (IVUS) of LCX; Double layer struct was BVS struct, but IVUS could not evaluate the condition of BVS clearly; (D) Optical coherence tomography (OCT) of LCX: good apposition of BVS.
DISCUSSION
BVS has the potential to revolutionize the percutaneous treatment of CAD. Unlike currently available drug-eluting stents (DESs), BVS do not remain in the vessel wall after their intended function of preventing acute recoil and limiting intimal hyperplasia is fulfilled.10 In a porcine coronary artery model, OCT struts appeared like a preserved box within two and half years, became open box between two and half years and three years, and dissolved black box between three years and three and a half years, and either dissolved bright box or were indiscernible within four years.11 In our patient, BVS struct still exist, which were not completely resolved in OCT image. The absorb time of the BVS struct may individualize by patients. According to the recent Absorb III, China, and Japan study, treating noncomplex obstructive CAD with a BVS was not inferior with respect to TLF, MI, cardiovascular mortality at 1-year compared with an everolimus-eluting stent.12-14 However, the use of BVSs to date has largely been restricted to patients recruited into clinical trials with a relatively small number of "real-world" patients treated with these devices. Here we explored the issue of BVS use in "real-world" patients, specifically in Taiwan.
BVS has been used to treat more complex lesions, such as in LM coronary arteries, bifurcated lesions, ostial lesions, calcified lesions, thrombus present, and CTOs in several recent cohort studies that reported MACE rates at 8.5%.15,16 Previous "real-world" outcomes of the BVS showed acceptable rates of TLR at six months. Diabetes mellitus was the only independent predictor of TLR.16 In our study, low MACE rate (0.6 %) and low scaffold thrombosis rates (0.6 %) occurred during the 30-day follow-up period. During the one-year follow-up period, MACE, definite scaffold thrombosis, and cardiovascular mortality rates were 5.1%, 1.3% and 1.3%, respectively.
Ostial lesions often have issues with instent restenosis (ISR), even after DES use. The BVS provided the opportunity to prevent stent-in-stent treatment, but also may increase the possibility of scaffold thrombosis. Other reasons for recurrent ISR after BVS implantation were related to newly developed neoatherosclerosis or inappropriate cessation of DAPT.17,18 Suboptimal implantation with incomplete lesion coverage, underexpansion, under deployment and malapposition comprised the primary mechanisms for both early and late BVS scaffold thrombosis, which is similar to metallic stent thrombosis. The rates of malapposition could be significantly improved by 1:1 pre-dilatation under OCT pullback analysis in recent studies.19 Unlike metallic stents, compliance chart information should not be used to predict final BVS dimensions in a clinical setting which was approved in a bench study.20 However, Wiebe et al. and other real-world cohort studies mentioned about the procedural learning curve such as post-dilatation and intravascular image guide, and could improve the clinical outcome during BVS implantation.21-23 However, in our study, we had a relatively high percentage of intravascular imaging use, and did not have an association between intravascular imaging use and one-year TLF. Puricel et al. also published the result of BVS-specific implantation strategy, which could significantly reduce the 12-month stent thrombosis rate from 3.3% to 1.0%.24 DAPT discontinuation also seemed to be a secondary contributor in several later events.25 According to one meta-analysis study, compared with a DES, patients undergoing PCI with a BVS had an increased rate of a definite/probable ST and MI during the follow-up.26
In the field of BVS for CTO, the CTO-ABSORB pilot study demonstrated the safety and feasibility of BVS use in cases of CTO recanalization; a key factor in those cases involves appropriate lesion preparation.27 In our study, no MACEs occurred in those patients treated with BVSs for a CTO lesion. Treating patients with ISR remains a clinical and technical challenge. The use of either a DES or the drug-coated balloon (DCB) is recommended in these patients.28 Some case reports and case series ensured excellent immediate results, profound anti-stenotic efficacy, avoiding the need for implanting an additional metal layer in the vessel wall.29 In our study, only one case had a TLR and two cases had TVR in those patients with BVSs for ISR. Currently, no study has analyzed the results of BVSs for LM lesions. Unfortunately, data are limited in terms of the use of BVSs in coronary bifurcation lesions. Thus, little is known about the safety and feasibility of these procedures. An interim report of 435 patients in the ABSORB-EXTEND trial showed a higher incidence of post-procedural side branch occlusion compared to the Everolimus-Eluting Stent (6.0% vs. 4.1%; p = 0.09).28 This effect was more pronounced in those cases with small side branches having a reference vessel diameter < 0.5 mm.30 However, no prospective randomized clinical data with OCT imaging for different bifurcation stenting techniques are available.
In Absorb III, TLR incidence in China and Japan ranged 3.4% to 7.8%, respectively, and TVR was from 4.2% to 9.1%, respectively. In our study, the TLR incidence was 3.6% and TVR was 5.1% during the one-year period of follow-up. Capodanno et al. described that diabetes mellitus was the only independent predictor of TLR (hazard ratio 2.41, 95% CI: 1.28-4.53; p = 0.006) in the report of the early and midterm clinical outcomes of PCI with a BVS from the large multicenter GHOST-EU registry.15 In our study, we found diabetes, ostial lesion, bifurcation lesion, and non-standard DAPT were the strongly associated with one-year TLF. According to our results, we need to underscore the importance of DAPT after BVS implantation, and prevent BVS use for patients who could disrupt DAPT by themselves.
In the field of using a BVS for STEMI, some studies presented excellent results. However, scaffold thrombosis clustered mostly in the early phase.31,32 Giblett et al. confirmed the delay of early neointimal growth and structure coverage after BVS implantation under OCT observation in acute coronary syndrome patients which might explain the elevated stent thrombosis rate in the registry results.33 Azzalini et al. stated that scaffold thrombosis may be a result of the prothrombotic milieu of acute coronary syndrome coupled with an unfavorable peristructure rheology of BVSs that might promote scaffold thrombosis early after implantation, particularly if other concomitant risk factors are present.34 But, TROFI II study showed similar endothelial coverage compared to metallic everolimus eluting stents and BVS in STEMI at 6-month follow-up.35 In our study, only 1.3% of patients with STEMI and 10.3% of patients with NSTEMI received a BVS implantation due to concerns of scaffold thrombosis in the period of a high thrombus burden environment, or if the vessel size could not be evaluated during the emergent condition if preparation could not be adequately performed during the critical condition.
Recently, more surgical professionals are attempting BVS implantation for complex lesions, calcified lesions, and more critical conditions. When increasing complexity of lesions in real-world practice, Liang HW et al. also reported 5.3% of MI, 2.6% ischemia-driven TLR, and 2.6% of non-fatal probable scaffold thrombosis.36 Therefore, we need to understand the limitations of BVSs, and those precautions associated with BVS implantation and "real-world" outcomes. According to our data and experience, BVSs are feasible for simple and complex lesions. A low definite and possible scaffold thrombosis rate was observed if there was appropriate stent preparation.
Limitations
Our study did have certain limitations. First, our investigation was observational in nature. However, it did provide important experience with BVS implantation in "real-world" practice, specifically in Taiwan. Second, our study had a relatively short follow-up period and absence of a control group. Third, our analysis for predictors of 1-year TLF was very exploratory. Some selection bias may have been present during the patient selection for BVS implantation. Even though our study cohort was small, we provided information about the importance of standard DAPT use after BVS implantation. Nonetheless, large-scale clinical data are necessary to explore the long-term benefit and clinical outcomes of BVS implantation.
CONCLUSIONS
Our investigation indicated that, even in difficult and complex lesions, acceptable outcomes were achieved with low definite or possible scaffold thrombosis rates following the implantation of BVS. Furthermore, despite of the presence of anatomical issues, it remains important to complete standard DAPT after BVS implantation.
SOURCES OF FUNDING
The first 40 patients were enrolled in ABSORB EXTEND trial and were supported by Abbott company.
DISCLOSURES
None.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
HUMAN RIGHTS STATEMENTS AND INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national), and with the Helsinki Declaration of 1964 and later revisions. Informed consent was obtained from all patients before being included in the study.
REFERENCES
- 1.Serruys PW, Onuma Y, Garcia-Garcia HM, et al. Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention. 2014;9:1271–1284. doi: 10.4244/EIJV9I11A217. [DOI] [PubMed] [Google Scholar]
- 2.Felix C, Everaert B, Diletti R, et al. Current status of clinically available bioresorbable scaffolds in percutaneous coronary interventions. Neth Heart J. 2015;23:153–160. doi: 10.1007/s12471-015-0652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ormiston JA, Serruys PW, Regar E, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899–907. doi: 10.1016/S0140-6736(08)60415-8. [DOI] [PubMed] [Google Scholar]
- 4.Serruys PW, Onuma Y, Ormiston JA, et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation. 2010;122:2301–2312. doi: 10.1161/CIRCULATIONAHA.110.970772. [DOI] [PubMed] [Google Scholar]
- 5.Ielasi A, Cortese B, Varricchio A, et al. Immediate and midterm outcomes following primary PCI with bioresorbable vascular scaffold implantation in patients with ST-segment myocardial infarction: insights from the multicentre "Registro ABSORB Italiano" (RAI registry). EuroIntervention. 2015;11:157–162. doi: 10.4244/EIJY14M10_11. [DOI] [PubMed] [Google Scholar]
- 6.Costopoulos C, Naganuma T, Latib A, et al. Optical coherence tomography of a bifurcation lesion treated with bioresorbable vascular scaffolds with the "minicrush" technique. JACC Cardiovasc Interv. 2013;6:1326–1327. doi: 10.1016/j.jcin.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Naganuma T, Costopoulos C, Latib A, et al. Feasibility and efficacy of bioresorbable vascular scaffolds use for the treatment of in-stent restenosis and a bifurcation lesion in a heavily calcified diffusely diseased vessel. JACC Cardiovasc Interv. 2014;7:e45–e46. doi: 10.1016/j.jcin.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 10.Latib A, Costopoulos C, Naganuma T, et al. Which patients could benefit the most from bioresorbable vascular scaffold implant: from clinical trials to clinical practice. Minerva Cardioangiol. 2013;61:255–262. [PubMed] [Google Scholar]
- 11.Brie D, Penson P, Serban MC, et al. Bioresorbable scaffold - a magic bullet for the treatment of coronary artery disease? Int J Cardiol. 2016;215:47–59. doi: 10.1016/j.ijcard.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Ellis SG, Kereiakes DJ, Metzger DC, et al. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med. 2015;373:1905–1915. doi: 10.1056/NEJMoa1509038. [DOI] [PubMed] [Google Scholar]
- 13.Gao R, Yang Y, Han Y, et al. Bioresorbable vascular scaffolds versus metallic stents in patients with coronary artery disease. J Am Coll Cardiol. 2015;66:2298–2309. doi: 10.1016/j.jacc.2015.09.054. [DOI] [PubMed] [Google Scholar]
- 14.Kimura T, Kozuma K, Tanabe K, et al. A randomized trial evaluating everolimus-eluting absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J. 2015;36:3332–3342. doi: 10.1093/eurheartj/ehv435. [DOI] [PubMed] [Google Scholar]
- 15.Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice:early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015;10:1144–1153. doi: 10.4244/EIJY14M07_11. [DOI] [PubMed] [Google Scholar]
- 16.Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the Absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention. 2015;10:1160–1168. doi: 10.4244/EIJY14M08_08. [DOI] [PubMed] [Google Scholar]
- 17.Bastante T, Rivero F, Benedicto A, et al. Recurrent neoatherosclerosis after bioresorbable vascular scaffold treatment of in-stent restenosis. JACC Cardiovasc Interv. 2015;8:1264–1265. doi: 10.1016/j.jcin.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Lee WC, Fang HY, Fang CY. Late stent thrombosis after the use of a bioresorbable vascular scaffold for the treatment of in-stent restenosis. Coron Artery Dis. 2016;27:709–710. doi: 10.1097/MCA.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 19.Brown AJ, McCormick LM, Braganza DM, et al. Expansion and malapposition characteristics after bioresorbable vascular scaffold implantation. Catheter Cardiovasc Interv. 2014;84:37–45. doi: 10.1002/ccd.25378. [DOI] [PubMed] [Google Scholar]
- 20.Attizzani GF, Ohno Y, Capodanno D, et al. New insights on acute expansion and longitudinal elongation of bioresorbable vascular scaffolds in vivo and at bench test: a note of caution on reliance to compliance charts and nominal length. Catheter Cardiovasc Interv. 2015;85:E99–E107. doi: 10.1002/ccd.25645. [DOI] [PubMed] [Google Scholar]
- 21.Wiebe J, Liebetrau C, Dorr O, et al. Impact of the learning curve on procedural results and acute outcome after percutaneous coronary interventions with everolimus-eluting bioresorbable scaffolds in all-comers population. Cardiovasc Revasc Med. 2015;16:455–460. doi: 10.1016/j.carrev.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Costopoulos C, Crowson MC, Brown AJ, et al. Mid-term clinical outcomes of ABSORB bioresorbable vascular scaffold implantation in a real-world population: a single-center experience. Cardiovasc Revasc Med. 2015;16:461–464. doi: 10.1016/j.carrev.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Robaei D, Back LM, Ooi SY, et al. Everolimus-eluting bioresorbable vascular scaffold implantation in real world and complex coronary disease: procedural and 30-day outcomes at two Australian centers. Heart Lung Circ. 2015;24:854–859. doi: 10.1016/j.hlc.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Puricel S, Cuculi F, Weissner M, et al. Bioresorbable coronary scaffold thrombosis: multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J Am Coll Cardiol. 2016;67:921–931. doi: 10.1016/j.jacc.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Karanasos A, Van Mieghem N, van Ditzhuijzen N, et al. Angiographic and optical coherence tomography insights into bioresorbable scaffold thrombosis: single-center experience. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipinski MJ, Escarcega RO, Baker NC, et al. Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2016;9:12–24. doi: 10.1016/j.jcin.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Vaquerizo B, Barros A, Pujadas S, et al. Bioresorbable everolimus-eluting vascular scaffold for the treatment of chronic total occlusions: CTO-ABSORB pilot study. EuroIntervention. 2015;11:555–563. doi: 10.4244/EIJY14M12_07. [DOI] [PubMed] [Google Scholar]
- 28.Alfonso F, Byrne RA, Rivero F, et al. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63:2659–2673. doi: 10.1016/j.jacc.2014.02.545. [DOI] [PubMed] [Google Scholar]
- 29.Rivero F, Bastante T, Cuesta J, et al. Treatment of in-stent restenosis with bioresorbable vascular scaffolds: optical coherence tomography insights. Can J Cardiol. 2015;31:255–259. doi: 10.1016/j.cjca.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Muramatsu T, Onuma Y, García-García HM, et al. Incidence and short-term clinical outcomes of small side branch occlusion after implantation of an everolimus-eluting bioresorbable vascular scaffold. JACC Cardiovasc Interv. 2013;6:247–257. doi: 10.1016/j.jcin.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Diletti R, Karanasos A, Muramatsu T, et al. Everolimus-eluting bioresorbable vascular scaffolds for treatment of patients presenting with ST-segment elevation myocardial infarction: BVS STEMI first study. Eur Heart J. 2014;35:777–786. doi: 10.1093/eurheartj/eht546. [DOI] [PubMed] [Google Scholar]
- 32.Brugaletta S, Gori T, Low AF, et al. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: the BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv. 2015;8:189–197. doi: 10.1016/j.jcin.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Giblett JP, Brown AJ, Keevil H, et al. Implantation of bioresorbable vascular scaffolds following acute coronary syndrome is associated with reduced early neointimal growth and strut coverage. EuroIntervention. 2016;12:724–733. doi: 10.4244/EIJV12I6A117. [DOI] [PubMed] [Google Scholar]
- 34.Azzalini L, L'Allier PL. Bioresorbable vascular scaffold thrombosis in an all-comer patient population: single-center experience. J Invasive Cardiol. 2015;27:85–92. [PubMed] [Google Scholar]
- 35.Sabate M, Windecker S, Iniguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J. 2016;37:229–240. doi: 10.1093/eurheartj/ehv500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang HW, Kao HL, Lin YH, et al. Everolimus-eluting bioresorbable vascular scaffold in real world practice - a single center experience. Acta Cardiol Sin. 2017;33:250–257. doi: 10.6515/ACS20160901A. [DOI] [PMC free article] [PubMed] [Google Scholar]