FIG 3.
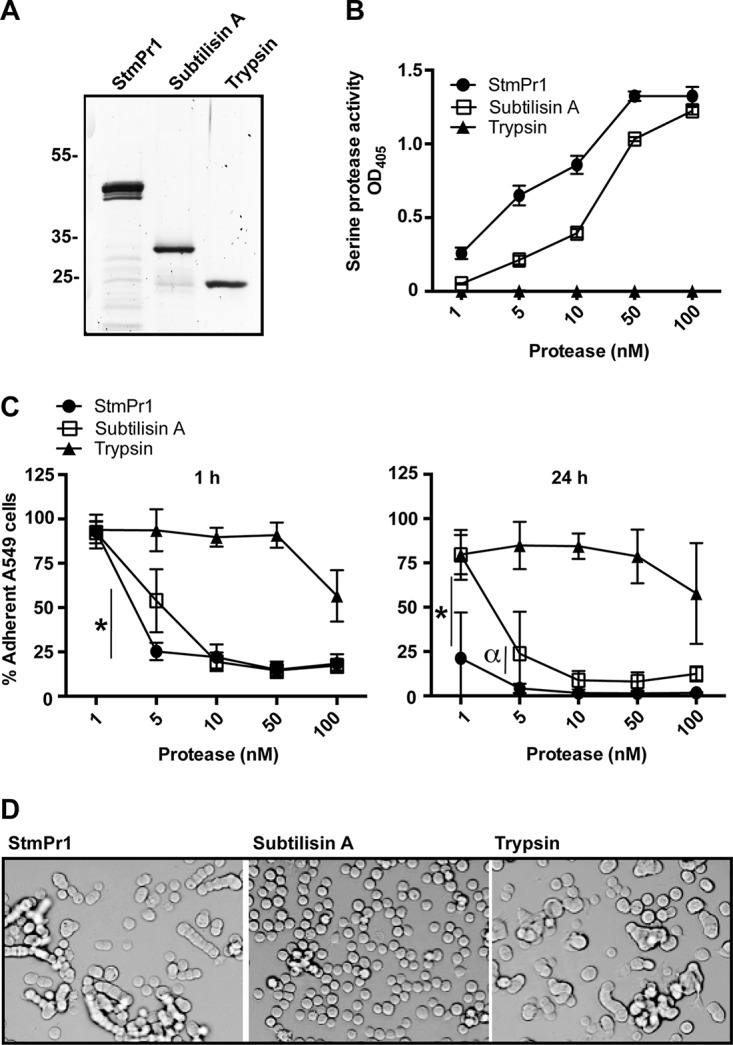
Characterization of the proteolytic and cell-detaching activities of purified StmPr1 compared to those of subtilisin A and trypsin. (A) StmPr1 was purified from the supernatant of the stmPr1 stmPr2 stmPr3 mutant complemented with stmPr1 using benzamidine-Sepharose. Purified StmPr1, subtilisin A, and trypsin (0.5 μg of each) were analyzed by SDS-PAGE, followed by SYPRO Ruby staining. (B) Purified StmPr1, subtilisin A, or trypsin was incubated with N-succinyl–Ala–Ala–Pro–Phe–pNA at the indicated equimolar concentrations for 60 min at 37°C to evaluate serine protease activity. The result for StmPr1 was statistically significantly different from the results for both subtilisin A and trypsin at all doses (P < 0.001). (C) A549 cells were incubated for 1 h or 24 h with the indicated equimolar concentrations of purified StmPr1, subtilisin A, or trypsin. Cell detachment was determined as described in the legend to Fig. 1. *, the difference between subtilisin A and StmPr1 was statistically significant (P < 0.001); α, the difference between subtilisin A and StmPr1 was statistically significant (P = 0.002). The results for both StmPr1 and subtilisin A were statistically significantly different from those for trypsin at the 5 to 100 nM doses (P < 0.001), and the results between StmPr1 and trypsin were also statistically significantly different at the 1 nM dose at 24 h (P < 0.001). (D) The morphology of A549 cells after 24 h of incubation with 100 nM StmPr1, subtilisin A, or trypsin was determined by phase-contrast light microscopy. Data are representative of those from three independent experiments. For panels B and C, data are represented as the mean and SD from three independent experiments.
