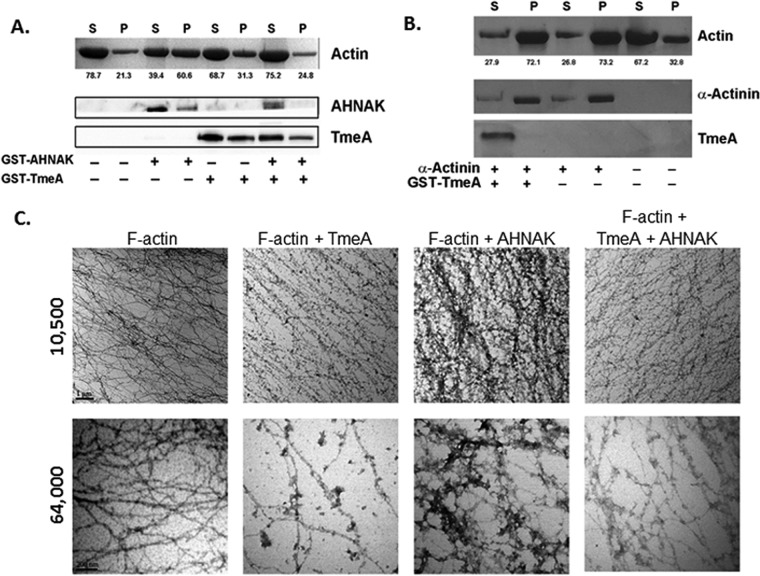
FIG 7.
TmeA inhibits AHNAK-dependent F-actin bundling activity in vitro. Rabbit skeletal muscle F-actin was subjected to low-speed (12,000 × g) differential centrifugation in the presence or absence of GST-tagged or control proteins. All recombinant proteins were mixed at 1:1 molar ratios. Samples subsequently were separated into supernatant (S) and pellet (P) fractions. Equal volumes of material were resolved by SDS-PAGE, and actin was visualized by Coomassie blue staining. (A) GST-AHNAK and GST-TmeA were detected by immunoblotting with AHNAK-specific or TmeA-specific antibodies and visualized via chemiluminescence after probing with secondary antibodies coupled to HRP. Numerical values represent the relative percentage of actin in respective fractions. (B) Actin, α-actinin, and GST-TmeA were visualized via Coomassie blue staining. (C) F-actin alone or in combination with GST-694 and/or GST-AHNAK in a 1:1 or 1:1:1 molar ratio was processed for visualization by transmission electron microscopy. Samples were visualized at 10,500× and 64,000× magnification, and representative images are shown. Scale bar, 1 μm.