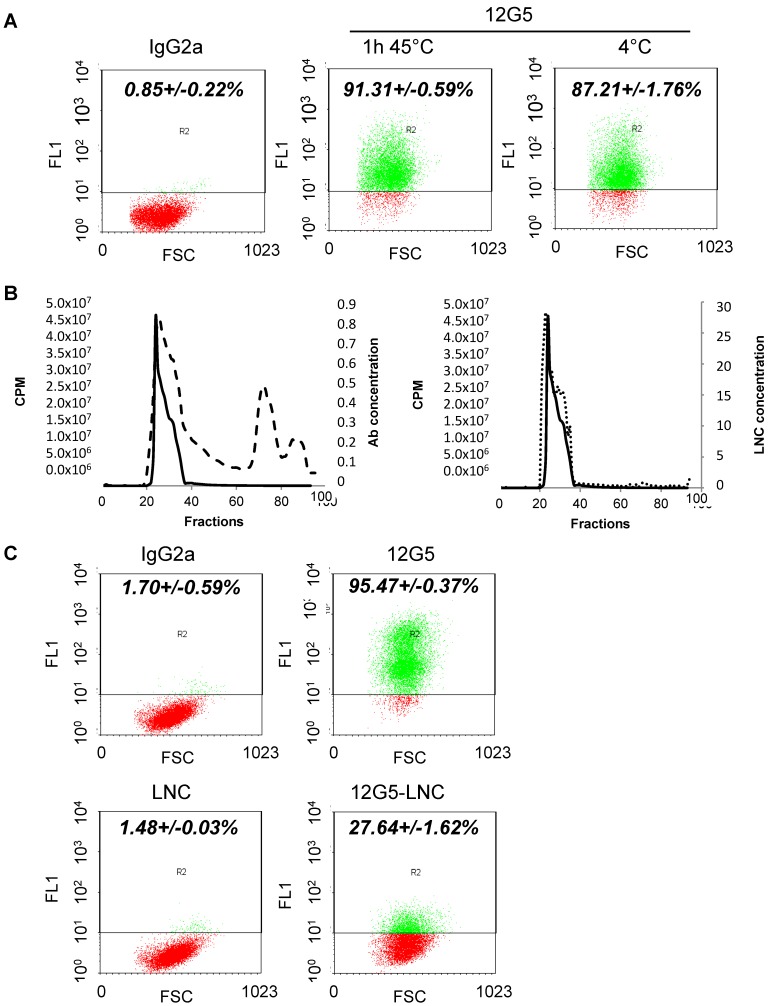
Figure 1.
Synthesis of lipid nanocarriers and bio-physical-chemical characterization. A) Stability of recognition of the 12G5 antibody depending on temperature assessed by flow cytometry analysis. B) Validation of immuno-LNC synthesis. Thiolated antibodies (12G5 and IgG2a) were added to LNC188Re or blank LNCs and incubated for 1 h at 45°C. Then, immuno-LNCs were purified on a sepharose column and positive fractions were concentrated. As represented on the left graph, micro-BCA assay was performed on each collected fraction to determine Ab concentration (dashed line) while gamma counting allowed measurement of the activity of each fraction (cpm, solid line). The right graph is representing LNC concentration determined by turbidimetric measurements (dotted line). C) Immunospecificity assay of free 12G5 antibody and 12G5-LNCs determined by flow cytometry.