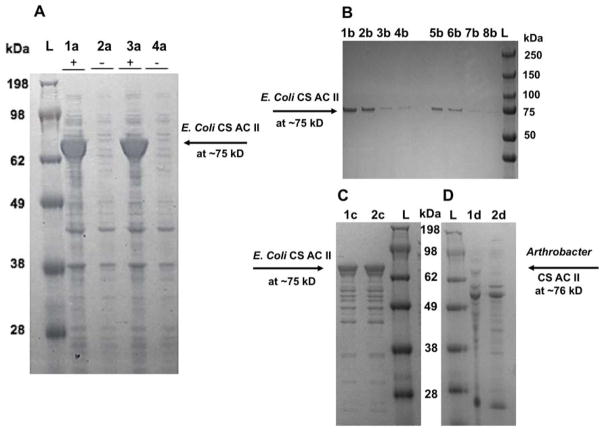
Figure 3.
The SDS-PAGE analysis of chondroitinase ACII enzymes with the ladder labeled L for each gel. Panel A gel result shows the induced and uninduced fractions of the tA16ACII and tA16ACII(I236T) enzymes expressed in E. coli BL21. The theoretical molecular weight was predicted to be 83.5 kDa and in the gel, the approximate molecular weight is ~75 kDa. The lanes are - induced tA16ACII(I236T): 1a, uninduced tA16ACII(I236T): 2a, induced tA16ACII: 3a, uninduced tA16ACII: 4a. Panel B gel result shows the tA16ACII and tA16ACII(I236T) enzymes expressed in E. coli BL21 and purified from Nickel column, with the tA16ACII(I236T) enzyme in the lanes on the left side of the gel and tA16ACII on the right side. The lanes are – 1b & 5b: soluble fraction, 2b & 6b: insoluble fraction, 3b & 7b: flow through, 4b & 8b: first wash. Panel C shows the gel result of the purified recombinant enzymes after buffer exchange in a 10 kDa spin column, with the lanes labeled – 1c: tA16ACII, 2c: tA16ACII(I236T). Panel D gel result shows the A16ACII enzyme (which has a theoretical molecular weight of ~76 kDa), among other proteins in the crude supernatant. The lanes are labeled – 1d: A16ACII grown in LB supplemented with 0.2% CS-A: A and 2d: A16ACII grown in LB supplemented with 0.2% CS-C.