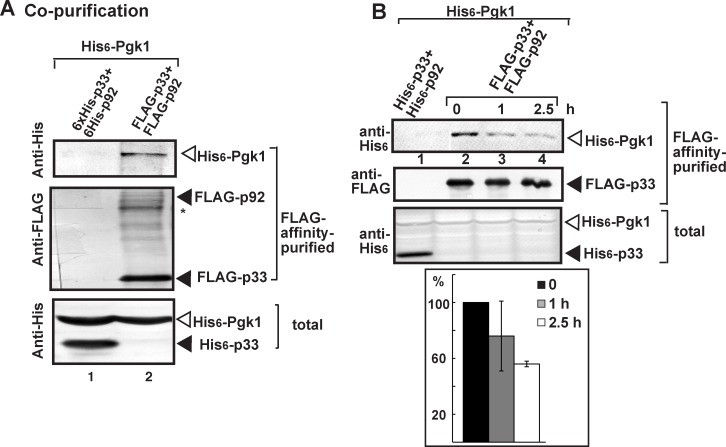
Fig 1. Interaction between p33 and p92pol replication proteins and yeast cytosolic Pgk1.
(A) Co-purification of Pgk1 with the viral replicase. Top panel: Western blot analysis of co-purified His6-tagged Pgk1 with FLAG-affinity purified FLAG-p33 and FLAG-p92pol from membrane fraction of yeast. Pgk1 was detected with anti-His antibody. The negative control was His6-tagged p33 and His6-p92 purified from yeast extracts using a FLAG-affinity column. Middle panel: Western blot of purified FLAG-p33 and FLAG-p92pol detected with anti-FLAG antibody. Asterisk marks the SDS-resistant p33 homodimer. Bottom panel: Western blot of His6-tagged Pgk1 and His6-p33 (lane 1) proteins in the total yeast extracts using anti-His antibody. (B) Testing the presence of Pgk1 in the membrane-bound viral replicase after blocking cellular translation by cycloheximide. Top panel: Western blot analysis shows the co-purified His6-tagged Pgk1 with the viral replicase isolated from membrane fraction at the shown time points. See further details in panel A. Middle panel: Western blot analysis of the purified FLAG-p33 with anti-FLAG antibody. Bottom panel: Western blot analysis of His6-Pgk1 in the total yeast lysates with anti-His antibody. Each experiment was repeated three times.