Figure 2. Overview of CAR T-cell therapy in the clinic.
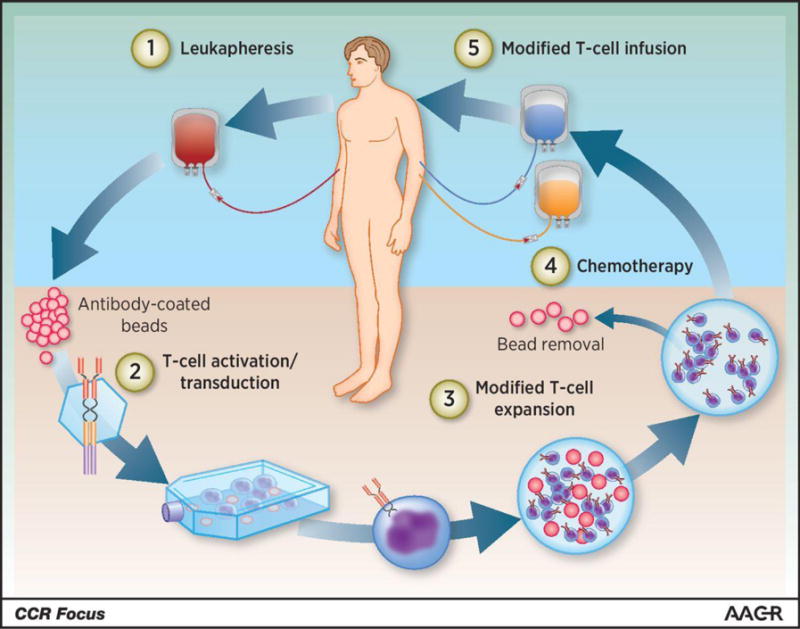
A patient’s T cells are harvested through leukapheresis, followed by T-cell activation on antibody-coated beads serving as artificial dendritic cells. The activated T cells are then genetically reprogrammed ex vivo by transduction with a construct encoding the CAR, and the CAR T cells are further expanded ex vivo. When the CAR T-cell product has been prepared and has passed all quality control testing, the patient receives lymphodepleting chemotherapy and CAR T-cell infusion. Adapted from Clin. Cancer Res. 22 (8), 1875–1884 (2016).18
