Figure 2. Proposed mechanism for the metallaphotoredox-mediated cross-coupling of carboxylic acids to generate sp3–sp3 bonds.
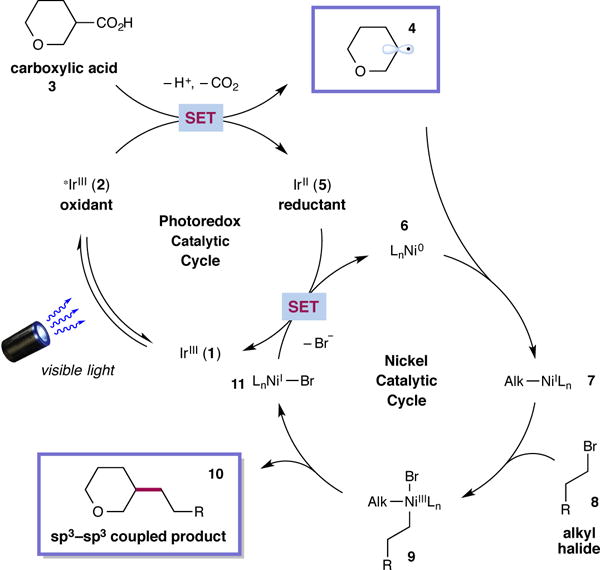
The photoredox catalytic cycle commences with excitation of IrIII 1 to give the excited state 2. Single electron oxidation of the carboxylate anion derived from acid 3 produces the alkyl radical 4 after CO2-extrusion along with IrII 5. Ni0 catalyst 6 captures the alkyl radical 4 to form the NiI species 7. Ensuing oxidative addition with alkyl halide 8 leads to nickel(III) intermediate 9. Reductive elimination would then liberate the desired product 10 and NiI 11. Both catalytic cycles converge to complete a single turnover via a SET event that regenerates the photoredox and nickel catalysts
