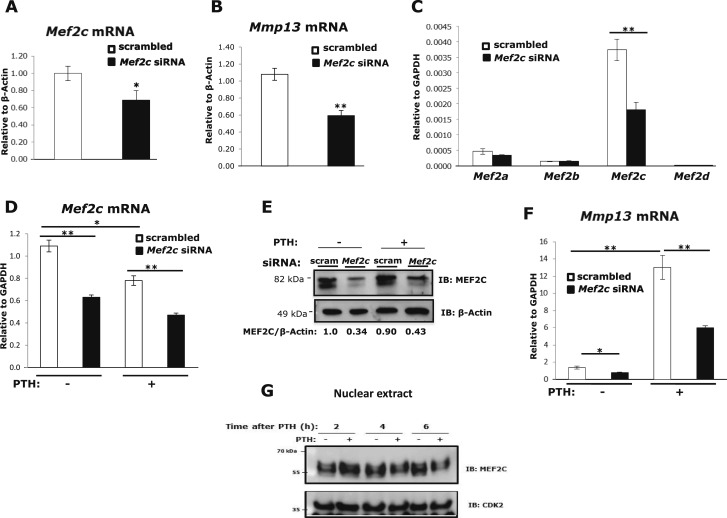
Figure 1.
MEF2C is required for Mmp13 gene expression and PTH regulates Mef2c. Primary mouse osteoblastic cells were transfected with 60 nM Mef2c siRNA or scrambled siRNA. Total RNA was isolated and amplified via real-time reverse transcription PCR. Relative levels of (A) Mef2c or (B) Mmp13 mRNAs (mean ± SE) were normalized to β-actin. *P < 0.05, **P < 0.01 vs scrambled control, n = 3. (C) Expression of Mef2 RNAs after Mef2c knockdown. UMR 106-01 cells were transfected with 60 nM Mef2c siRNA or scrambled siRNA. Seventy-two hours after transfection, relative levels of the mRNAs (mean ± SE) were normalized to Gapdh. **P < 0.01 vs scrambled control, n = 3. (D) UMR 106-01 cells were transfected with Mef2c siRNA or scrambled siRNA. Cells were treated with 10−8 M PTH-(1-34) for 4 hours and collected for RNA analysis. Relative levels of Mef2c mRNA (mean ± SE) were normalized to Gapdh. *P < 0.05, **P < 0.01 vs control or PTH-treated scrambled set, n = 3. (E) Western blot of MEF2C. Cells were treated with PTH for 30 minutes and whole cell lysates were collected for protein expression analysis. Protein samples were separated using 8% SDS-PAGE and immunoblotted with antibodies against MEF2C and β-actin as indicated. (F) After transfection, cells were treated with PTH for 4 hours and collected for RNA analysis. Relative levels of Mmp13 mRNA were normalized to Gapdh. Data shown are mean ± SE. *P < 0.05, **P < 0.01 vs control or PTH-treated scrambled set, n = 3. (G) UMR 106-01 cells were treated with or without PTH at indicated times and nuclear extracts were collected for protein expression. Protein samples were separated using 8% SDS-PAGE and immunoblotted with antibodies against MEF2C and CDK2 as indicated.