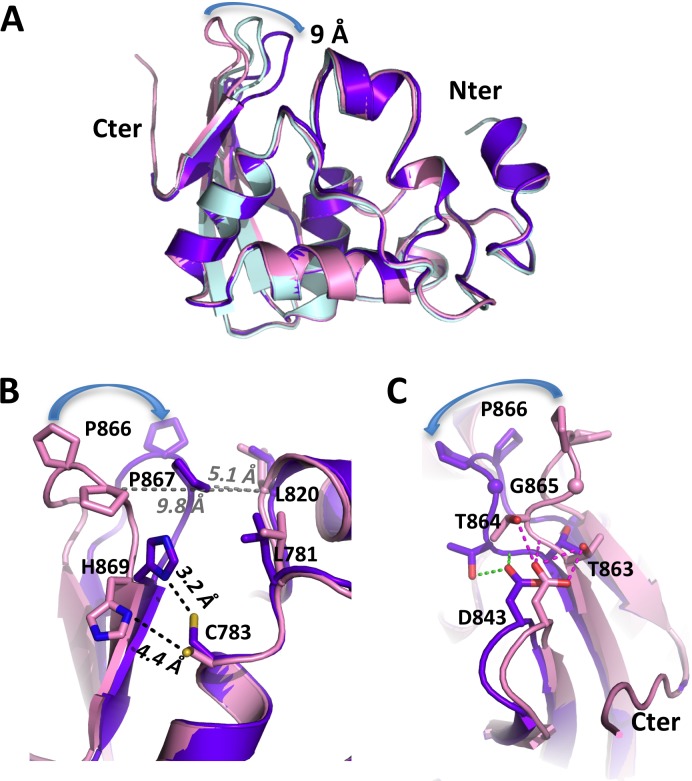
Fig 1. Crystal structure of TYMV PRO/DUB mutant ΔC5.
(A) Superposition of PRO/DUB wild type (in pink) and ΔC5 ('A' and 'B', in light cyan and purple-blue, respectively). The displacement of loop 864–868 (865-GPP-867 flap) is indicated with a blue arrow following P866. (B) Superposition of the two most distant conformations (wild type and ΔC5 'B' conformation) seen from the P' side of the catalytic cleft. Residues forming the hydrophobic zipper, as well as the catalytic dyad C783-H869, are displayed as sticks and labeled. The distances between the Sγ of C783 and the Nδ1 of H869 are indicated in black and the distances between the Cαs of P867 and L820 in gray. The displacement of P866 in the zipper is indicated by a blue arrow as in panel (A). (C) Same superposition as in (B) seen from the other side of the GPP flap. Note that D843 follows the movement of the neighbouring GPP loop but that some of its interactions with the base of the loop break.