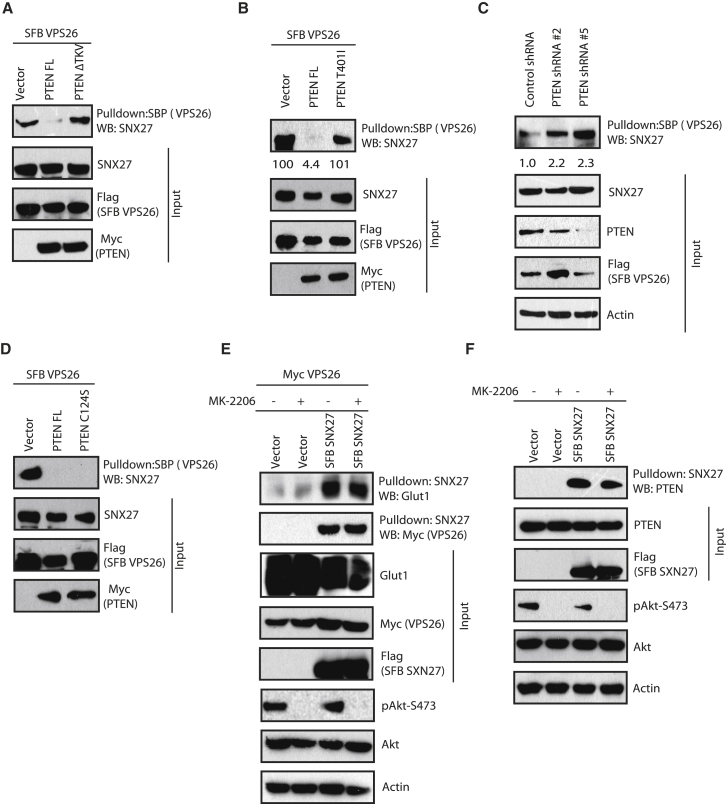
Figure 5.
PTEN Disrupts SNX27-VPS26 Association Independent of Its Catalytic Activity
(A and B) SFB-tagged VPS26 along with Myc vector, Myc-tagged PTEN FL, or the PTEN ΔTKV mutant (A) or the PTEN T401I mutant (B) was co-transfected in HEK293T cells, and cell lysates were subjected to pull-down with streptavidin beads. The interaction of VPS26 with SNX27 was determined by western blotting with endogenous SNX27 antibody. The inputs were blotted with the respective antibodies.
(C) HeLa cells transduced with either control or PTEN shRNAs were transfected with SFB-tagged VPS26, and the cell lysates were subjected to pull-down with streptavidin beads. Interaction of VPS26 with SNX27 was determined by western blotting with endogenous SNX27 antibody.
(D) HEK293T cells were co-transfected with SFB-tagged VPS26 along with Myc- tagged PTEN FL or the catalytically inactive PTEN C124S mutant. Cell lysates were pulled down with streptavidin beads, and interaction was detected by immunoblotting with SNX27 antibodies.
(E) Cells were transfected with SFB vector or SFB-SNX27 along with Myc-VPS26. 24 hr after transfection, cells were treated with an Akt inhibitor (MK-2206, 10 μM) for 4 hr. Cells lysates were subjected to pull-down with streptavidin beads, and western blotting was performed using specific antibodies.
(F) Cells were transfected with SFB vector or SFB-SNX27 and treated with an Akt inhibitor. Cell lysates were subjected to pull-down with streptavidin beads, and endogenous interaction of PTEN was detected.