Abstract
The ability to interpret facial expressions of others is one of the more important abilities possessed by humans. However, is it possible for humans to accurately interpret the facial expressions of another species of primate, namely rhesus monkeys (Macaca mulatta)? We investigated this possibility by taking digital photos of four rhesus monkeys housed either singly or socially and allowing thirty-one participants to judge these photographs as representing either a happy, sad, or neutral monkey. Results indicated that the photographs of monkeys that were socially housed were more likely to be rated as happy or neutral than were photographs of singly housed monkeys. We suggest that these results imply important parallels between the perception of human and nonhuman primate facial expressions as well as introduce a potential new method for assessing nonhuman primate well-being.
Keywords: monkey, face, countenance, enrichment, housing, judgment
Humans are particularly adept at interpreting information conveyed by facial expressions (Parr, Hopkins & deWaal, 1998). Terrestrial primates, who in general rely on visual communication signals for survival, greatly benefit from “understanding” faces. Therefore, facial expressions become especially important if we consider the valuable information they provide about the identity, intentions, social status, and underlying emotions of individuals who participate in complex social interactions.
The neural and cognitive processes involved in face perception by humans and other primates appear to be distinct from those processes that commonly take place in the visual perception of other objects (Carmel & Bentin, 2002). Indeed, considerable controversy exists as to whether this difference is due to the fact that faces comprise a specific domain for which some authors suggest an innate predisposition (Easterbrook, Kisilevsky, Hains & Muir, 1999), or if in fact the difference in processing is merely explained by the long and vast experience humans accumulate with facial stimuli during their life span (Gauthier, Skudlarski, Gore & Anderson, 2000; Gauthier, Tarr, Anderson, Skudlarski & Gore, 1999.). These two explanations notwithstanding, most researchers agree upon the special status held by human faces in the human visual processing system.
Interestingly, several researchers argue that other primate faces may also hold a similar processing advantage for humans. In other words, ape and monkey faces, but not other non-primate animal faces, would be affected by the well-known perceptual singularities reported for human faces, i.e. the inversion effect, left-side attentional bias, and categorical perception.
The inversion effect, or impairment of face recognition compared to other object recognition when stimuli are presented in an inversed orientation, has been reported in human adults (Campbell, Pascallis, Coleman, Wallace & Benson, 1997) and children (Pascalis, Demont, de Haan & Campbell, 2001) who had no previous systematic experience with monkey, sheep, or cow faces, when looking at human or rhesus monkey faces, but not when viewing sheep or cow faces. It is interesting to note that the inversion effect has also been reported for chimpanzees when looking at chimpanzee and human faces, but not when looking at capuchin faces (Parr, Dove,& Hopkins, 1998).
Similarly, Fernández-Carriba et al. (2002) found left attentional biases associated with processing of faces and emotions of chimpanzee (Pan troglodytes) faces in humans with and without chimpanzee experience, but only when the faces were presented in their normal orientation (vs. horizontally reversed). The perceptual advantage of facial and emotional stimuli placed on the left side of the observer has been thoroughly investigated in humans and other primates and has been interpreted as evidence of right hemisphere dominance in the perception of facial expression of emotions. In a previous study, Overman and Doty (1982) failed to find these attentional biases in humans when looking at rhesus monkey faces. However, these researchers also failed to find an attentional bias for rhesus monkeys when looking at the faces of conspecifics, a finding consistently obtained by later researchers (Hamilton & Vermeire 1983, 1988; Vermeire & Hamilton, 1988, 1998).
Finally, Campbell et al. (1997) used morphed image series of human, rhesus monkey, and bovine faces to test for categorical perception of the three species’ faces. Humans without experience with monkey and cow faces were able to make accurate judgments of the three categories. However, in a follow-up discrimination task, images that were far from the previous category boundaries were more easily discriminated only in the monkey-cow and human-cow morphs, but not in the monkey-human images. According to the authors, these findings suggested that only the distinction between primate faces and cow faces was categorically perceived and that humans might judge monkey faces in terms of human characteristics, albeit distinctive ones.
Therefore, similar face processing mechanisms in humans when looking at human and nonhuman primates are suggested by several investigators, however, little has been presented about what information, if any, nonhuman primate faces convey to inexperienced human observers. According to Hauser (1993) and Fernández-Carriba, Loeches, Morcillo, and Hopkins (2002) humans without previous experience with chimpanzees or rhesus monkeys seem to be sensitive to the emotional intensity of their facial expressions. These authors found that the assessments made by humans regarding the intensity of each half of a monkey or chimpanzee face during the display of an emotional expression was highly consistent with physical measures of facial asymmetry (e.g. both assessments indicated that the left side of the monkeys’ and chimpanzees’ faces were the most emotionally intense).
Additionally, humans with no previous chimpanzee experience may be able to identify correctly many chimpanzee facial expressions, as suggested by Ekman’s (1973) review of Foley’s (1935) classic study. Foley’s early work failed to demonstrate the ability of college students to identify accurately six chimpanzee facial expressions. Later work by Ekman (1973) with Foley’s original data revealed that the subjects, to a significant degree in fact, labeled five of the expressions above chance levels (two of the chimpanzee expressions had not been correctly identified by Foley and he had not performed statistics on his data). These later findings would seem to imply that the naïve human observers in this study approached chimpanzee faces in ways similar to human faces, but also that the participants may have understood to some extent the emotions portrayed by those faces. To our knowledge, Foley’s findings have not been replicated, and no study to date has addressed the question as to whether or not facial communication could be possible between members of different species.
With this previous research in mind, we sought to determine to what extent human participants, with little or no previous experience with rhesus monkeys, were sensitive to the different emotional consequences that variations in housing condition (social vs. single) would potentially have on rhesus monkeys based solely upon the monkey’s facial expressions. Assuming the presence of special mechanisms for humans dealing with primate facial stimuli (human and nonhuman) we hypothesized that naïve observers would unknowingly be able to differentiate various housing conditions and even make an accurate assessment as to the monkey’s welfare in each condition.
Method
Participants
Thirty-one undergraduates (average age 19.77 years, 7 males) participated in exchange for course credit.
Materials and Procedure
Photographs of four male rhesus monkeys (Macaca mulatta, average age 14.75 years) were taken using a Casio digital camera (Model QV-10A). Twenty photographs were taken of each monkey in each of two housing conditions; singly-housed indoors, and socially-housed indoors with access to an outdoor play-yard. Photography sessions were randomized by time and subject over the course of one month. One photo was made per session. Monkeys in both housing conditions had continuous access to the Language Research Center’s Computerized Test system (LRC-CTS; see Rumbaugh, Savage-Rumbaugh, Washburn, Richardson, & Hopkins, 1989). The LRC-CTS has previously been shown to have numerous positive effects on these same monkeys’ psychological well-being with the monkeys engaging in fewer cage directed, self directed, and stereotypic behaviors (Washburn & Rumbaugh, 1992; Washburn, Rumbaugh, & Richardson, 1992).
The enclosures in the singly housed condition measured 90cm × 90cm × 180cm. Monkeys in the socially housed condition were paired with a compatible conspecific and housed in a 5.2m × 2.7m × 2.4m indoor enclosure with access to a 7.8m × 9.0m × 2.4m outdoor enclosure. Housing conditions were counterbalanced such that the first two monkeys were photographed in the socially housed condition during which time the other two monkeys were being photographed in the singly housed condition, conditions were then reversed. Each monkey’s housing conditioned remained constant for one week prior to being photographed. Special care was taken to insure that the distance of the monkeys from the camera (approximately 2 feet) was equivalent in all photographs. Additionally, the photographer was well known to the monkeys thereby insuring that the monkeys’ “safe distance” was not compromised during photography sessions. Threat displays were infrequent and photos of such displays were excluded from the final sample used for this investigation. In each photograph the monkey was facing the photographer ensuring that both of the monkey’s eyes were visible.
Each of the 160 photographs was edited using Photomagic version 1.0 (Micrografx, Allen, TX) such that only the head and small portions of the upper torso of the monkeys remained visible on a white background, thereby affording observers no information as to the housing condition (single or social) or body position of the monkey visible in each photo (image size was approximately 400 × 370 pixels). Examples of the actual photographs used are shown in Figure 1.
Figure 1.
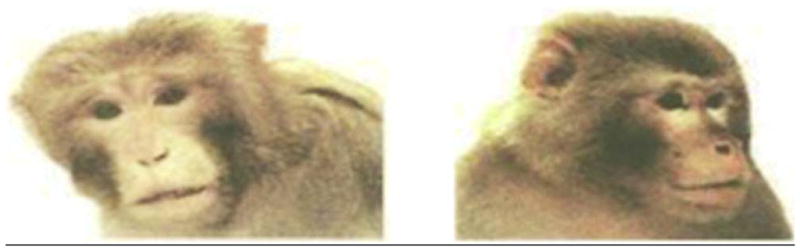
This figure contains examples of two of the photographs presented to the participants in this investigation. NOTE: Participants viewed these images in color.
Six photographs of each monkey in each condition were then selected at random. Adobe Photoshop 5.5 was used to determine the luminosity of the 48 photos. An independent measures t-test was conducted to determine whether the luminosity of the photographs varied as a function of the monkeys’ housing condition. Results indicated no significant difference in luminosity (t (17) = .39, p> .94). The 48 photos were then loaded into MS-PowerPoint (version 2007). Four PowerPoint presentations, containing each of the 48 photos presented in different orders, were created in which no two photos of the same animal or housing condition appeared consecutively.
Participants were seated in front of an IBM-compatible computer with a 15-inch monitor and instructed to use the left mouse button to scroll through 1 of the 4 MS PowerPoint presentations that had been selected for them at random by the experimenter. Participants were informed that they could view each of the photos in the presentation as long as they wished. Participants were provided with a pencil and instructed to rate the well-being of the monkey in each of the photographs based upon the monkey’s facial expression by circling one of three available responses on the rating sheet provided by the experimenter. The rating sheet consisted of numerals (1–48) corresponding to each of the photos in the presentation as well as three potential response selections for each of the photographs. The available response selections for each of the photographs were, HAPPY, NEUTRAL, or SAD.
Results
Ratings by 27 of the 31 participants’ were higher for animals in the socially housed condition. Proportionally, more of the photographs were rated as depicting a monkey with a happy or neutral countenance in the socially housed condition, whereas more of the photographs were rated as depicting a sad monkey in the singly housed condition (see Figure 2).
Figure 2.
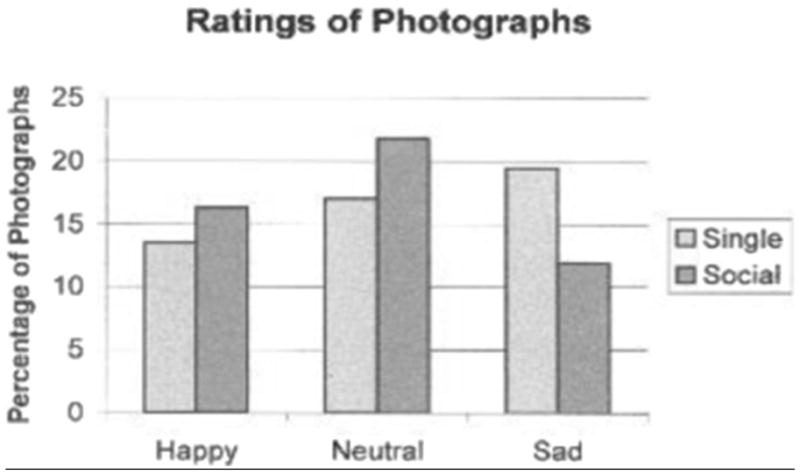
Percentage of the 48 photographs judged to be happy, sad, or neutral by the participants for each of the housing conditions.
Participants’ responses to each of the photographs were coded (Happy = 1, Neutral = 0, Sad = −1) and totaled for each of the two housing conditions. To ascertain whether participants’ responses varied as a function of housing condition of the monkeys depicted in the photographs a Wilcoxon signed-ranks test was conducted with significant results (z = −4.51, p < .01).
Additionally, in order to determine whether the same pattern of results (i.e., greater happiness judgments for the social versus non-social condition) was present for the individual monkeys, Wilcoxon signed-ranks tests were again computed. Results for ratings of photographs of the individual monkeys once again revealed significant differences based upon housing condition (see Table 1).
Table 1.
Results of Wilcoxon signed-ranks test for ratings of photographs of individual monkeys
p< .01
p< .05
Discussion
This study, to our knowledge, presents the first data whereby humans were asked to attempt to “interpret” and apply emotional states to rhesus monkeys based upon the information provided solely by facial cues. These humans were extremely consistent in their appreciation of the faces presented, i.e., 27 out of the 31 participants rated the monkeys as being “happier” when socially housed. Furthermore, the fact that this categorization matches the two external conditions (happy and socially housed; sad and individually housed) indicates that the judgments made by the observers were probably based on these underlying contingencies and not merely upon a random response pattern.
This interpretation is also upheld by all the behavioral evidence of a high specificity in human processing mechanisms when dealing with primate facial stimuli (human and nonhuman) (Carmel & Bentin, 2002). The rationale for such a competence with faces that do not belong to our species is necessarily connected with the similarities in facial morphology between the two species and, therefore, the inferences of the human observers in our study were likely anthropomorphic. Anthropomorphism would have been then a useful strategy for humans without previous experience with rhesus monkeys, who were nonetheless able to extract some information from the faces of the monkeys.
Faces of primate species would not only look similar, but they would also function similarly, according to morphological descriptions of the repertoire of facial expressions in humans, apes, and certain species of monkeys (Chevalier-Skolnikoff, 1973). Based on this premise, a common evolutionary history has been suggested for some of those facial expressions, i.e. they would be homologous. In the close connection between production and perception of facial stimuli, similarities in primate facial expression must be accompanied by face-processing mechanisms that are similar for primate species (see above). Within this context, it is not surprising that humans with no direct experience with other primate species might be capable of extracting some meaning from their faces, as in Foley’s (1935) study and in the findings reported here.
The fact that the most frequent category applied to the individually housed monkeys was “sad” is not trivial and warrants further discussion. Preuschoft (2000) has argued that the impression of a lonely or sad monkey is evoked primarily by slow movements and noticeably weak muscle tone, and not by a facial expression. Preuschoft also suggested that the evolution of displays of sadness is closely tied to the evolution of altruism; i.e., there must be a compassionate individual to perceive the signal for it to be effective. Our results indicate, however, that information merely provided by the face is enough to label a monkey as “sad”. The existence of a facial display of sadness in adult rhesus monkeys is not, however, a conclusion that can be reached without further investigation. The fact that these results occurred in the context of a forced-choice task is a clear limitation to these findings.
These results indicate that humans are capable of detecting differences in the countenance of monkeys housed in distinctive conditions. Participants rated monkeys that were socially housed as being significantly happier than when they were singly housed. Are singly housed monkeys unhappy monkeys? Not necessarily, such a statement cannot be made based solely on these data. But, in this case these particular monkeys were judged to be happier when socially housed by naive observers. As noted earlier, each of the monkeys depicted in the photographs utilized in this investigation had continuous access to the LRC-CTS. Interestingly, LRC-CTS performance for all monkeys remained high regardless of housing condition. The LRC-CTS has been shown to promote psychological well-being and enhance environmental enrichment (Washburn & Rumbaugh, 1992; Washburn, Rumbaugh, & Richardson, 1992). Therefore, we must conclude that the singly housed monkeys are not necessarily “sad,” insofar as they are possibly simply not as “happy” as the monkeys in the socially housed condition.
In conclusion, these results seem to indicate that it is possible for humans to make an accurate judgment of nonhuman primate well-being based solely on facial expressions. Such simple human responses may provide a new and effective method for making an assessment of nonhuman primate well-being.
Acknowledgments
This research was supported by NICHD grants HD38051 and HD060563 to Georgia State University and a Research Program Enhancement Grant from Georgia State University.
References
- Campbell R, Pascalis O, Coleman M, Wallace SB, Benson PJ. Are faces of different species perceived categorically by human observers? Proceedings of the Royal Society of London, Series B – Biological Sciences. 1997;264:1429–1434. doi: 10.1098/rspb.1997.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel D, Bentin S. Domain specificity versus expertise: Factors influencing distinct processing of faces. Cognition. 2002;83:1–29. doi: 10.1016/s0010-0277(01)00162-7. [DOI] [PubMed] [Google Scholar]
- Chevalier-Skolnikoff S. Facial Expressions of Emotion in Nonhuman Primates. In: Ekman P, editor. Darwin and facial expressions. Academic Press; New York: 1973. [Google Scholar]
- Easterbrook MA, Kisilevsky BS, Hains SJ, Muir DW. Faceness or complexity evidence from newborn visual tracking of face-like stimuli. Infant Behavior and Development. 1999;1:17–35. [Google Scholar]
- Ekman P. Darwin and facial expressions. New York: Academic Press; 1973. Cross-cultural studies of facial expression; pp. 169–222. [Google Scholar]
- Fernández-Carriba S, Loeches A, Morcillo A, Hopkins WD. Asymmetry in facial expression of emotions by chimpanzees. Neuropsychologia. 2002;40:1523–1533. doi: 10.1016/s0028-3932(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Fernández-Carriba S, Loeches A, Morcillo A, Hopkins WD. Asymmetry in facial expression of emotions by chimpanzees. Neuropsychologia, Vol. 2002;40(9):1523–1533. doi: 10.1016/s0028-3932(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Foley JP. Judgment of facial expression of emotion in the chimpanzee. Journal of Social Psychology. 1935;6:31–54. [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience. 1999;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle funiform “face area” increases with expertise in recognizing novel objects. Nature Neuroscience. 2000;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Hamilton CR, Verneire BA. Discrimination of monkey faces by split-brain monkeys. Behavioral Brain Research. 1983;9:263–275. doi: 10.1016/0166-4328(83)90132-8. [DOI] [PubMed] [Google Scholar]
- Hamilton CR, Verneire BA. Complimentary hemispheric specialization in monkeys. Science. 1988;242:1694–1696. doi: 10.1126/science.3201258. [DOI] [PubMed] [Google Scholar]
- Hauser MC. Right hemisphere dominance in the production of facial expressions in monkeys. Science. 1993;261:475–477. doi: 10.1126/science.8332914. [DOI] [PubMed] [Google Scholar]
- Overman WH, Doty RW. Hemispheric specialization displayed by man, but not macaques in the analysis of faces. Neuropsychologia. 1982;20:113–128. doi: 10.1016/0028-3932(82)90002-1. [DOI] [PubMed] [Google Scholar]
- Parr LA, Dove TA, Hopkins WD. Why faces may be special: Evidence of the inversion effect in chimpanzees (Pan troglodytes) and rhesus monkeys(Macaca mulatta) Journal of Cognitive Neuroscience. 1998;10:615–622. doi: 10.1162/089892998563013. [DOI] [PubMed] [Google Scholar]
- Parr LA, Hopkins WD, DeWaal FBM. The perception of facial expressions by chimpanzees, (Pan troglodytes) Evolution of Communication. 1998;2:1–23. [Google Scholar]
- Pascalis O, Demont E, de Haan M, Campbell R. Recognition of faces of different species: A developmental study between 5 and 8 years of age. Infantand Child Development. 2001;10:39–45. [Google Scholar]
- Preuschoft S. Primate faces and facial expressions. Social Research. 2000;67:245–271. [Google Scholar]
- Rumbaugh DM, Savage-Rumbaugh ES, Washburn DA, Richardson WK, Hopkins WD. Rhesus monkeys (Macaca mulatta), video tasks and implications for stimulus-response spatial contiguity. Journal of Comparative Psychology. 1989;103:32–38. doi: 10.1037/0735-7036.103.1.32. [DOI] [PubMed] [Google Scholar]
- Verneire BA, Hamilton CR. Laterality in monkeys discriminating inverted faces. Neuroscience Abstracts. 1988;14:1139. [Google Scholar]
- Vermeire BA, Hamilton CR. Inversion effect for faces in split- brain monkeys. Neurospychologia. 1998;36:1003–10014. doi: 10.1016/s0028-3932(98)00054-2. [DOI] [PubMed] [Google Scholar]
- Washburn DA, Rumbaugh DM. Investigation of rhesus monkey video-task performance: Evidence for enrichment. Contemporary Topics in Laboratory Animal Science. 1992;31:6–10. [PubMed] [Google Scholar]
- Washburn DA, Rumbaugh DM, Richardson WK. The Language Research Center’s computerized test system for environmental enrichment and Psychological assessment. Contemporary Topics in Laboratory Science. 1992;31:11–15. [PubMed] [Google Scholar]


