Figure 8. Model of the functional interplay between O-acetylation and transpeptidation.
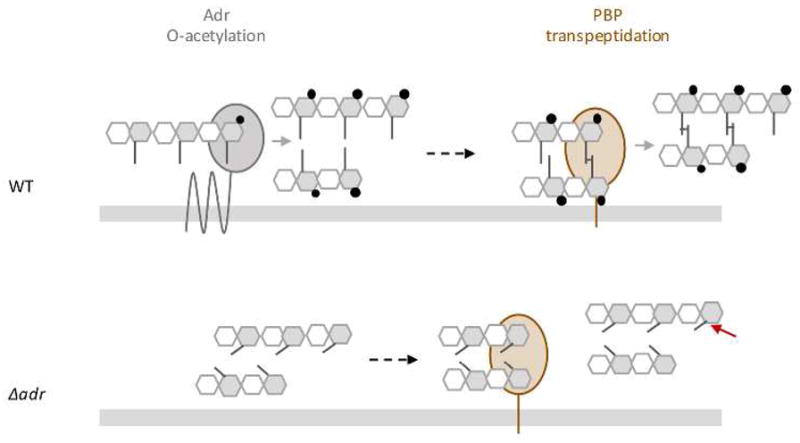
Adr protein which catalyzes the O-acetylation reaction is represented as a multimembrane protein (for details, see Fig S6A). Only one PBP is represented as a bitopic membrane protein with the catalytic domain exposed in the extracellular space. For clarity reason, the represented PBP only refers to the transpeptidation reaction, the glycan chain polymerization activity is not shown. The grey bar accounts for the cytoplasmic membrane. The peptidoglycan glycan chains are formed by the repetition of MurNAc and GlcNAc, grey and white hexagons, respectively. Peptide stems (grey lines) are linked to MurNAc and cross-linked to each other (transversal lines). O-acetylation of MurNAc residues is represented by black circles. Light grey arrows indicate O-acetylation and transpeptidation reactions. The dotted black arrow illustrates the fact that O-acetylation of MurNAc would precede peptides cross-linking in our working model. Non cleavage of the amide bond by LytA in O-acetylation peptidoglycan is indicated by a diamond red arrow, while cleavage when peptidoglycan is O-de-acetylated is represented by a red arrow.
In dividing WT cells where exponential growth and peptidoglycan synthesis take place (upper panel), Adr O-acetylates MurNAc residues on glycan strands before transpeptidation to produce mature peptidoglycan. In such a structure, the amide bond is not accessible, impeding its cleavage by LytA and thus conferring resistance towards cell lysis. The absence of Adr (Δadr strain) induces alteration of the peptidoglycan structure, which in turns affects the transpeptidation efficiency, increases the sensitivity to LytA cleavage (the amide bond cleavage site is indicated by the red arrow), alters the cell morphology and impacts the division process (the latter two features are not represented in the figure).
