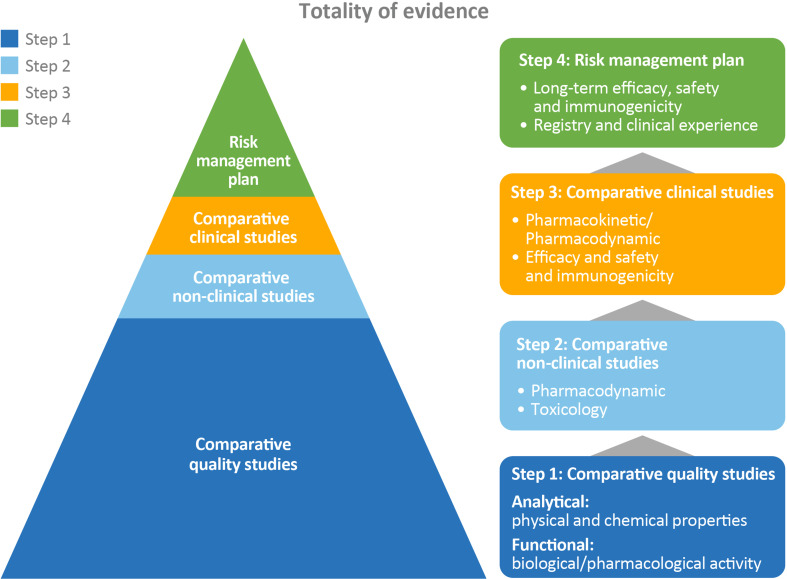
Fig. 1.
Data requirements for approval of a biosimilar [14]. Regulatory agencies in the EU and USA have defined a pathway for the development of a biosimilar that is designed to leverage the existing information and clinical experience from the reference product, resulting in reduced clinical trials for the biosimilar candidate and a greater preponderance of analytical characterization as well as non-clinical and clinical pharmacology data. In order for the biosimilar candidate to leverage the clinical history of the reference product, the biosimilar must demonstrate analytically similarity to the reference product. The higher the similarity at the analytical level, the lower the uncertainty will be that the biosimilar will behave differently at the clinical level. This is a stepwise approach to establishing biosimilarity where each step must be satisfied as no steps can refute significant differences in the preceding steps.
Adapted from Biosimilars in the EU. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Leaflet/2017/05/WC500226648.pdf (Accessed August 2017)