Abstract
Prolonged farrowing remains one of the critical challenges in intensive pig farming. This study aims to explore the effects and mechanism of Yimu San (YMS), a Chinese veterinary medicine micro mist, on delivery ability with mouse models. Thirty-two pregnant mice were randomly divided into a control group and low-YMS, med-YMS, and high-YMS groups. The labor process time and stillbirth rate were recorded, the levels of serum oxytocin and prostaglandin E2 (PGE2) were measured with enzyme-linked immunosorbent assay (ELISA). Contractility measurements of the isolated uterus and the expression of connexin 43 (Cx43) in uterine smooth muscle were evaluated. The results showed that compared with the control group, the birth process time and stillbirth rate in the med-YMS and high-YMS groups were remarkably lower. The in vitro uterine contractions, levels of oxytocin, PGE2, and Cx43 in the med-YMS and high-YMS groups were significantly higher than those in the control group. The differences of the above measurements between the low-YMS group and the control group were not obvious. It can be speculated that YMS could significantly promote labor in pregnant mice by enhancing the levels of oxytocin, Cx43, and PGE2.
Keywords: Yimu San, Delivery ability, Uterine contraction, Oxytocin, Prostaglandin E2, Connexin 43
1. Introduction
Prolonged farrowing remains one of the critical challenges in intensive pig farming. It not only is a detriment to the sows but also increases the mortality of piglets (Oliviero et al., 2010). Borges et al. (2005) found that sows with prolonged farrowing (more than 3 h) had a 2-fold increase in the risk of stillbirth. Prolonged farrowing is associated with lower oxytocin levels (Alonso-Spilsbury et al., 2004). Oxytocin is a neuropeptide which comprises nine amino acids, and it is mainly synthesized in the hypothalamus and released from the neurohypophysis into the blood circulation. Oxytocin has a clear effect in promoting uterine contractions during parturition, and has a significant role in inducing labor (Devost and Zingg, 2007; Flenady et al., 2014). It is involved in the onset and maintenance of spontaneous labor (Kuwabara et al., 1987; Frey et al., 2015). In many commercial pig farms worldwide, oxytocin is used to decrease the duration of farrowing, but the mortality rate of piglets and the mean interval between successive piglets might increase (Gilbert, 1999; Mota-Rojas et al., 2002; Alonso-Spilsbury et al., 2004). Therefore, some research suggested that oxytocin should be forbidden in the farrowing induction of current pig production (Holtz et al., 1990).
Traditional Chinese veterinary medicine is the treasure of Chinese traditional medicine. With the rapid development of modem animal husbandry, it has become an important topic in recent years. Traditional Chinese veterinary medicine has the advantages of little drug resistance and drug residue, minimal side effects, and ease of use. Yimu San (YMS) (Wang et al., 2016) is a Chinese veterinary medicine micro mist developed by the Agricultural Biological Products and Seed Industry Zhongguancun Opening Laboratory of Beijing University of Agriculture, China. It is mainly composed of the monarch drug Leonurus japonicus Houut. and the ministerial drug Angelica sinensis. The function of YMS is to benefit vital energy and nourish the blood, invigorate blood circulation and relieve pain, increase uterine contractions, relieve uterine inertia, and reduce the duration of parturition. Previous research (Wang et al., 2016) found that YMS can significantly shorten labor time in sows and reduce piglet mortality. However, the mechanisms by which it exerts its effects remain unclear.
Uterine contraction is regulated by several factors, such as oxytocin, prostaglandin E2 (PGE2), connexin, progesterone, and estrogen. Connexin 43 (Cx43) is an important part of the gap junction protein and is widely distributed in the organs and tissues, particularly expressed in the myocardium and uterus (Contreras et al., 2003). Cx43 can form channels for the direct movement for small molecules between adjacent cells (Jiang et al., 2007) or between the intracellular and extracellular environments (Goodenough and Paul, 2003; Plotkin and Bellido, 2013) in the cytomembrane. Cx43 levels are low in the myometrium of the nonpregnant uterus, but increase significantly just before the onset of labor and then disappear shortly after delivery (Orsino et al., 1996; Ou et al., 1997), which indicates that the expression of Cx43 in the myometrium is temporary and strictly associated with parturition.
PGE2 also has significant roles in the labor process. It can stimulate uterine contractions, and lead to fetal membrane rupture as well as promote cervical ripening and dilatation (Olson, 2003). The actions of PGE2 are mediated by four subtypes of prostaglandin E receptor EP1‒4 (Morsy et al., 2001). They were discovered in uterine smooth muscle, cervix, amnion, and placenta (Astle et al., 2005; Unlugedik et al., 2010). The prostaglandins released in these tissues are involved in uterine contraction and female reproduction, which indicates the close correlation with labor.
In the present study, the effects of YMS on the labor process time and stillbirth rate, coupled with the ability of uterine contraction, the levels of oxytocin, Cx43, and PGE2 were monitored in the puerperal mice. We wanted to explore the possible mechanisms of the positive influence of YMS on labor, and establish whether there is reliable experimental evidence supporting the wide application of YMS in animal husbandry.
2. Materials and methods
2.1. Preparation of Yimu San
The traditional Chinese herb micro mist YMS was decocted by 10-fold volume of water three times, 1.5 h each time. Then the filtrate was collected and concentrated to paste with a relative density of 1.20–1.25 (50 °C). The paste was dried and crushed, and blended with an appropriate amount of starch. Finally, YMS was sub-packaged and stored for experimental purposes. The active ingredient of YMS is leonurine hydrochloride and the content is 74.79 µg/g.
2.2. Experimental design
A total of 48 healthy CD1 mice (32 females and 16 males) were purchased from the Merial Vital Laboratory Animal Technology Co., Ltd. (Beijing, China). They were caged in a female to male ratio of 2:1 for mating. Successful fertilization was confirmed by the observation of vaginal plug and this day was regarded as the 0.5 d of gestation. Pregnant mice were randomly divided into four groups (n=8 in each group): in the control group, the mice were treated with physiological saline; in the low-YMS group, YMS was given in the low dosage of 1.08 g/kg body weight; in the med-YMS group, YMS was given in the medium dosage of 3.25 g/kg body weight; in the high-YMS group, YMS was given in the high dosage of 6.50 g/kg body weight. From 16 d pregnancy to delivery, all mice in different groups were injected with physiological saline or fed with YMS once a day. The birth process time, the number in the litter, and the stillbirth of each mouse were recorded. The study was approved by the Beijing Municipal Committee of Animal Management and the Ethics Committee of China Agricultural University, China. All the experimental procedures were in accordance with the institutional criteria for the care and use of laboratory animals.
2.3. Blood sampling and the measurement of oxytocin and PGE2
Mice were sacrificed at 0.5 h after labor by intraperitoneal injection of 3% (0.03 g/ml) pentobarbital sodium (100 mg/kg). Blood was obtained from the eyeball, allowed to stand for 30 min, then centrifuged at 10 000 r/min for 5 min. The supernate was separated and stored at −20 °C. Oxytocin and PGE2 levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (NJJCBio Inc., Nanjing, China) according to the manufacturer’s instructions.
2.4. Contractility measurements
Laparotomy was performed at the abdominal midline to separate the uterus. The uterus was removed immediately and the left uterine horn was obtained. Uteri were placed longitudinally in the smooth muscle experimental system (HW-200S, Taimeng Software Pty. Ltd., Chengdu, China) at constant temperature. The system contained Krebs-Ringer bicarbonate (KRB) solution with the following composition: 120 mmol/L NaCl, 4.6 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 1.5 mmol/L CaCl2, 20 mmol/L NaHCO3, and 11 mmol/L glucose KRB solution (pH 7.4). It was maintained at 37 °C and continuously gassed with a mixture of 95% O2 and 5% CO2. Each uterus was placed under a resting force of 1 g and allowed to equilibrate for 0.5 h. The uterine contractions were recorded with the tension transducer (FT-100, Taimeng Software Pty. Ltd., Chengdu, China) which was connected to a Powerlab bridge (BL-420F, Taimeng Software Pty. Ltd.). Chart for Windows 7 software was used to display and analyze the tension changes in the tissue.
2.5. Western blotting analysis for Cx43
Western blotting was used to analyze uterine smooth muscle Cx43 protein expression levels of different groups. The samples were homogenized with protein extraction reagents (78510; Pierce Biotechnology Inc., Waltham, MA, USA). The homogenates were mixed with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; P0015, Beyotime Institute of Biotechnology, Jiangsu, China). The mixture was boiled, then cooled in an ice bath and centrifuged. Protein lysates were separated by 10% (0.10 g/ml) SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% (0.05 g/ml) skimmed milk, then incubated overnight with primary antibodies of Cx43 (ab11370; Abcam, Cambridge, UK; 1:5000 (v/v)) and glycralehyde-3-phosphate dehydrogenase (GAPDH; ab9485; 1:100 (v/v)) at 4 °C. GAPDH served as the internal reference. Blots were then incubated with horseradish peroxidase (HRP)-conjugated goat antibody to mouse immunoglobulin G (IgG; CW0102, Beijing ComWin Biotech Co., Ltd., Beijing, China; 1:2000 (v/v)). After adequate washing, PVDF membranes were finally incubated with Clarity™ Western ECL Substrate (170-5060; Bio-Rad Laboratories Inc., Hercules, CA, USA), and the immunoreactive proteins were visualized with the use of an imaging system (VersaDoc; Bio-Rad, USA).
2.6. Immunohistochemical staining
Paraffin sections were incubated with rabbit polyclonal antibody against Cx43 (ab11370; Abcam; 1:2000 (v/v)). A biotinylated donkey antibody to rabbit IgG (SP9002; Zhongshan Golden Bridge Biotechnology, Beijing, China; 1:200 (v/v)) and HRP-conjugated streptavidin (SP9002; Zhongshan Golden Bridge Biotechnology) were added. Subsequently, sections were treated with 3,3'-diaminobenzidine-4HCl and H2O2 (ZLI-9017; Zhongshan Golden Bridge Biotechnology) and counterstained with hematoxylin. The optical density of Cx43 in different groups was measured using Image-Pro Plus 6.0.
2.7. Statistical analysis
The data were analyzed with SPSS software, Version 17.0 (SPSS Inc., Chicago, IL, USA). Continuous data were given as mean±standard deviation (SD) and analyzed by one-way analysis of variance (ANOVA) and multiple comparisons. The difference of stillbirth rate was determined with the Chi-square test. A value of P<0.05 was considered statistically significant.
3. Results
3.1. Effects of YMS on birth process time and stillbirth rate in each group
The birth process duration in the med-YMS and high-YMS groups was remarkably shorter than that in the control group (P<0.05), whereas the time was relatively unchanged between the control and low-YMS groups (Fig. 1a). The high-YMS and med-YMS groups displayed quite a lower stillbirth rate than the control group (P<0.05). However the rate did not show any significant differences between the low-YMS group and the control group (Fig. 1b).
Fig. 1.
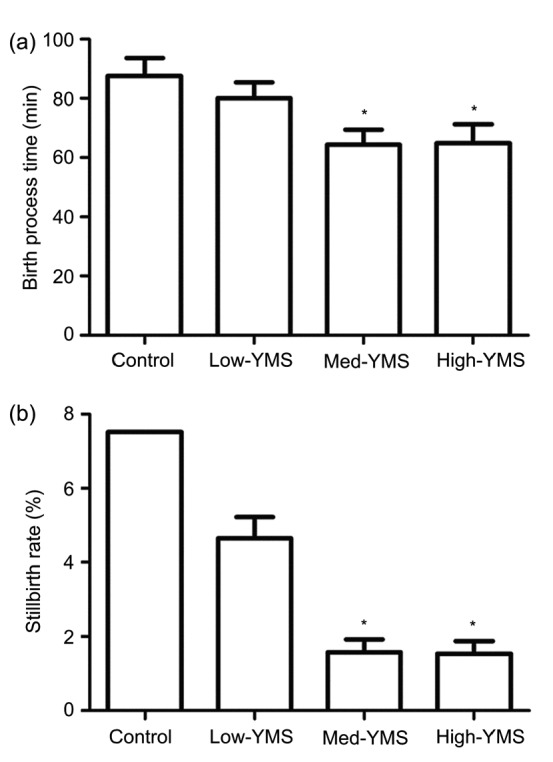
Effects of YMS on the labor process time (a) and stillbirth rate (b) in each group
Data are expressed as mean±SD (n=8). * P<0.05 vs. the control group
3.2. Effect of YMS on contractions of uterine smooth muscle
Fig. 2 displays the recordings of the myometrial contractions in mice treated with different doses of YMS (0, 1.08, 3.25, and 6.50 g/kg body weight) in vitro. The results show that the amplitude and muscle tension of uterine contraction in the med-YMS and high-YMS groups were remarkably higher than those in the control group (P<0.05), whereas there was no significant difference between the low-YMS group and the control group (Figs. 2b and 2c). No statistical difference was observed in the frequency of uterine contraction between each group (Fig. 2d).
Fig. 2.
Effect of YMS on the contractions of uterine smooth muscle
(a) Spontaneous rhythmic activity of uterine in different groups. (b) The amplitude of uterine contraction in different groups. (c) The average muscle tension of uterine contraction in different groups. (d) The frequency of uterine contraction in different groups. Data are expressed as mean±SD (n=8). * P<0.05 vs. the control group
3.3. Effects of YMS on serum oxytocin and PGE2 levels
Compared with the control group, the use of YMS yielded markedly higher levels of serum oxytocin in the YMS-treated group (P<0.05), and the oxytocin levels increased with the dose of YMS. As for serum PGE2, the med-YMS and high-YMS groups had significantly higher levels than the control group, while the difference between the low-YMS and control groups was not significant (Fig. 3).
Fig. 3.

Effects of YMS on serum oxytocin and PGE2 levels
Data are expressed as mean±SD (n=8). # P<0.05 vs. the oxytocin level in the control group; * P<0.05 vs. the PGE2 level in the control group
3.4. Effect of YMS on the expression levels of Cx43 in uterine smooth muscle
Expression of Cx43 in uterine smooth muscle was evaluated by Western blotting (Fig. 4). Cx43 expression was remarkably higher in both the med-YMS and high-YMS groups when compared with the control group (P<0.05), but there were no significant differences between the low-YMS group and the control group.
Fig. 4.

Effect of YMS on the expression level of Cx43 in uterine smooth muscle
Expression of Cx43 in uterine smooth muscle was evaluated by Western blotting. Data are expressed as mean±SD (n=6). * P<0.05 vs. the control group
3.5. Effect of YMS on Cx43 immunoreactivity in myometrium
Fig. 5 shows the immunohistochemistry for Cx43 in the uterine tissue of puerperal mice. Positive results are displayed in brown-colored staining in smooth muscle cells. Cx43 immunoreactivity was distributed predominantly in the cytoplasm around the smooth muscle cell nucleus and cytomembrane (Fig. 5a). Compared with the control group, the med-YMS and high-YMS groups had a higher optical density of Cx43 (Fig. 5b). Consistent with the above results, Cx43 immunoreactivity in the low-YMS group was relatively unchanged.
Fig. 5.
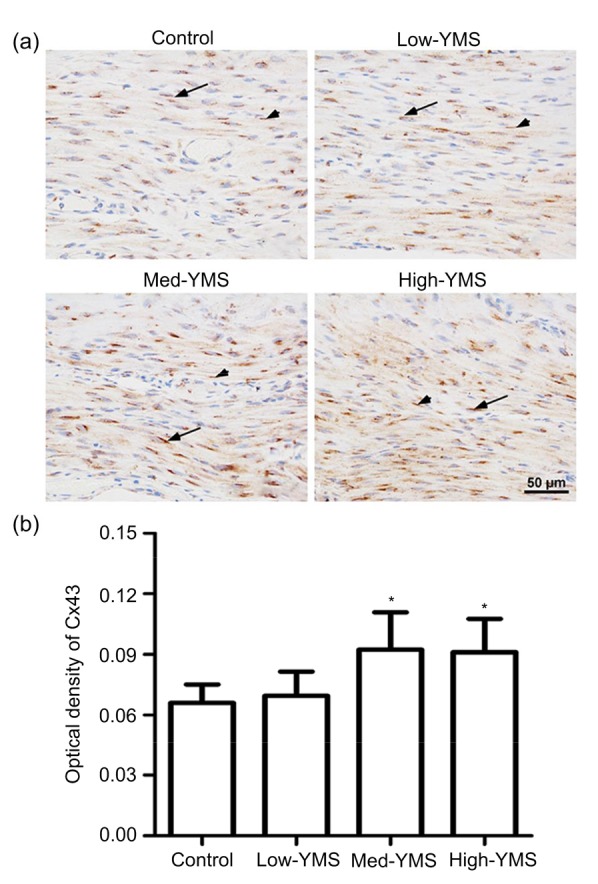
Effect of YMS on Cx43 immunoreactivity in myometrium
(a) Immunohistochemistry showed that cytoplasmic Cx43 was distributed around the smooth muscle nucleus (arrows) and cytomembrane (arrowheads). Blue indicates the cell nucleus. (b) Optical density of Cx43 in each group. Data are expressed as mean±SD (n=6). * P<0.05 vs. the control group (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
4. Discussion
Prolonged farrowing always causes a high stillbirth rate, and piglet mortality represents an economic loss to intensive pig farming. The long duration of farrowing deserves a lot of attention. Different from monotocous animals, in pigs, as polytocous animals, the use of oxytocin may decrease the duration of farrowing, but the mortality rate in their offspring remains unchanged (González-Lozano et al., 2009). Mice and pigs have many similarities. Both are polytocous animals. Compared with pigs, mice have obvious research advantages of rapid growth and reproduction. Thus, this study investigated the influence of a newly developed Chinese veterinary medicine micro mist, YMS, on labor using mouse models.
The results showed that the birth process time and stillbirth in the med-YMS and high-YMS groups were remarkably lower than those in the control group. The in vitro uterine contractions, levels of oxytocin, PGE2, and Cx43 in the med-YMS and high-YMS groups were significantly higher than those of the control group. The differences of the above measurements between the low-YMS group and the control group were not obvious. All these phenomena demonstrated that YMS can have a prominent role in the promotion of labor, and its positive influence was more obvious with increased dosage.
Our previous clinical research (Wang et al., 2016) showed that YMS could improve the delivery ability of sows. In the present study, YMS significantly increased the oxytocin levels in mice. It could be speculated that the improved delivery ability of pregnant mice might come from the increased endocrine oxytocin levels which were regulated by YMS, and the endocrine oxytocin levels had a better and more continuous effect on promoting labor than exogenous oxytocin.
PGE2 is a type of prostaglandin and is widely used in clinically induced labor (Keirse, 2006). Zhou et al. (2010) demonstrated that the Chinese herbal medicine Yimu Shenghua San could increase PGE2 levels and placental hormone levels in retained placenta cows. This study showed that YMS significantly increased the PGE2 content in mouse serum. There existed a certain correlation between PGE2 and oxytocin in the decidua and epithelial tissue of the uterus. Oxytocin could stimulate the synthesis and release of PGE2 and prostaglandin F2α, which further increased the susceptibility of uterus to oxytocin and thus strengthened subsequent uterine contractions (Chan et al., 2004). Therefore, YMS might promote PGE2 production via the increased serum oxytocin levels.
Smooth labor requires a highly coordinated and strongly contracting uterus. The intensity, frequency, and duration of uterine contractions are of great importance for the successful expulsion of the progeny (Kota et al., 2013). In this study, the medium and high doses of YMS significantly improved the amplitude and muscle tension of uterine contractions, but had no significant effect on the frequency. YMS might improve the delivery ability of pregnant mice by enhancing the amplitude and muscle tension of uterine contractions.
The expression of Cx43 in cervical muscle cells increased rapidly in full-term pregnancy and parturition. This significant change enhanced the electric coupling of uterine smooth muscle cells (Bruzzone 2001; Maass et al., 2004). Sometimes, due to the biological factors or human intervention, a relatively reduced expression of Cx43 can lead to a delayed delivery (Cluff et al., 2006; Tong et al., 2009). Western blotting analysis demonstrated that, compared with the control group, Cx43 expression in mouse uterine smooth muscle of the med-YMS and high-YMS groups was significantly increased. Cx43 was observed in the cytoplasm around the smooth muscle nucleus and cytomembrane by immunohistochemistry, and the positive expression intensity of Cx43 in the med-YMS and high-YMS groups was enhanced significantly, which supported the data obtained from Western blotting experiments. Thus, YMS might enhance uterine contractions through the up-regulation of Cx43 in uterine smooth muscle cells.
5. Conclusions
In conclusion, the present study showed that YMS might improve the delivery ability of pregnant mice via increasing the levels of oxytocin and Cx43 in uterine smooth muscle, as well as enhancing PGE2 concentrations. Future work will study the binding of oxytocin on myometrial membranes, the molecular mechanism, and the site of action. Overall, our findings provide an experimental principle for the clinical application of YMS.
Contributors
Study concept and design: Yun-fei MA and Ke-dao TENG; acquisition of data: Qi-huan WANG, Shuang ZHANG, and Li-meng QIN; statistical analysis: Qi-huan WANG and Wen-jun ZHANG; drafting of the article: Qi-huan WANG and Yun-fei MA; administrative, technical, and material support: Yun-fei MA, Ke-dao TENG, Feng-hua LIU, and Jian-qin XU.
Footnotes
Project supported by the Public Service Sectors Agriculture Research Projects of Ministry of Agriculture of China (No. 201403051-07), the National Natural Science Foundation of China (No. 31502025), and the Chinese Universities Scientific Fund (No. 2015DY003)
Compliance with ethics guidelines: Qi-huan WANG, Shuang ZHANG, Li-meng QIN, Wen-jun ZHANG, Feng-hua LIU, Jian-qin XU, Yun-fei MA, and Ke-dao TENG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Alonso-Spilsbury M, Mota-Rojas D, Martínez-Burnes J, et al. Use of oxytocin in penned sows and its effect on fetal intra-partum asphyxia. Anim Reprod Sci. 2004;84(1-2):157–167. doi: 10.1016/j.anireprosci.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Astle S, Thornton S, Slater DM. Identification and localization of prostaglandin E2 receptors in upper and lower segment human myometrium during pregnancy. Mol Hum Reprod. 2005;11(4):279–287. doi: 10.1093/molehr/gah158. [DOI] [PubMed] [Google Scholar]
- 3.Borges VF, Bernardi ML, Bortolozzo FP, et al. Risk factors for stillbirth and foetal mummification in four Brazilian swine herds. Prev Vet Med. 2005;70(3-4):165–176. doi: 10.1016/j.prevetmed.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Bruzzone R. Learning the language of cell-cell communication through connexin channels. Genome Biol. 2001;2(11):reports4027. doi: 10.1186/gb-2001-2-11-reports4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan LY, Fu L, Leung TN, et al. Obstetric outcomes after cervical ripening by multiple doses of vaginal prostaglandin E2 . Acta Obstet Gynecol Scand. 2004;83(1):70–74. doi: 10.1111/j.1600-0412.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- 6.Cluff AH, Byström B, Klimaviciute A, et al. Prolonged labour associated with lower expression of syndecan 3 and connexin 43 in human uterine tissue. Reprod Biol Endocrinol. 2006;4:24–32. doi: 10.1186/1477-7827-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contreras JE, Sáez JC, Bukauskas FF, et al. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA. 2003;100(20):11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devost D, Zingg HH. Novel in vitro system for functional assessment of oxytocin action. Am J Physiol Endocrinol Metab. 2007;292(1):E1–E6. doi: 10.1152/ajpendo.00529.2005. [DOI] [PubMed] [Google Scholar]
- 9.Flenady V, Reinebrant HE, Liley HG, et al. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst Rev. 2014;6:CD004452. doi: 10.1002/14651858.CD004452.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frey HA, Tuuli MG, England SK, et al. Factors associated with higher oxytocin requirements in labor. J Matern Fetal Neonatal Med. 2015;28(13):1614–1619. doi: 10.3109/14767058.2014.963046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert GL. Oxytocin secretion and management of parturition in the pig. Reprod Domest Anim. 1999;34(3-4):193–200. doi: 10.1111/j.1439-0531.1999.tb01240.x. [DOI] [Google Scholar]
- 12.González-Lozano M, Mota-Rojas D, Velázquez-Armenta EY, et al. Obstetric and fetal outcomes in dystocic and eutocic sows to an injection of exogenous oxytocin during farrowing. Can Vet J. 2009;50(12):1273–1277. [PMC free article] [PubMed] [Google Scholar]
- 13.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4(4):285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 14.Holtz W, Schmidt-Baulain R, Meyer H, et al. Control of prostaglandin-induced parturition in sows by injection of the β-adrenergic blocking agent carazolol or carazolol and oxytocin. J Anim Sci. 1990;68(12):3967–3971. doi: 10.2527/1990.68123967x. [DOI] [PubMed] [Google Scholar]
- 15.Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–1462. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keirse MJNC. Natural prostaglandins for induction of labor and preinduction cervical ripening. Clin Obstet Gynecol. 2006;49(3):609–626. doi: 10.1097/00003081-200609000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Kota SK, Gayatri K, Jammula S, et al. Endocrinology of parturition. Indian J Endocrinol Metab. 2013;17(1):50–59. doi: 10.4103/2230-8210.107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwabara Y, Takeda S, Mizuno M, et al. Oxytocin levels in maternal and fetal plasma, amniotic fluid, and neonatal plasma and urine. Arch Gynecol Obstet. 1987;241(1):13–23. doi: 10.1007/BF00931436. [DOI] [PubMed] [Google Scholar]
- 19.Maass K, Ghanem A, Kim JS, et al. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin 43. Mol Biol Cell. 2004;15(10):4597–4608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morsy MAM, Isohama Y, Miyata T. Prostaglandin E2 increases surfactant secretion via the EP1 receptor in rat alveolar type II cells. Eur J Pharmacol. 2001;426(1-2):21–24. doi: 10.1016/S0014-2999(01)01211-0. [DOI] [PubMed] [Google Scholar]
- 21.Mota-Rojas D, Martínez-Burnes J, Trujillo-Ortega ME, et al. Effect of oxytocin treatment in sows on umbilical cord morphology, meconium staining, and neonatal mortality of piglets. Am J Vet Res. 2002;63(11):1571–1574. doi: 10.2460/ajvr.2002.63.1571. [DOI] [PubMed] [Google Scholar]
- 22.Oliviero C, Heinonen M, Valros A, et al. Environmental and sow-related factors affecting the duration of farrowing. Anim Reprod Sci. 2010;119(1-2):85–91. doi: 10.1016/j.anireprosci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003;17(5):717–730. doi: 10.1016/S1521-6934(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 24.Orsino A, Taylor CV, Lye SJ. Connexin-26 and connexin-43 are differentially expressed and regulated in the rat myometrium throughout late pregnancy and with the onset of labor. Endocrinology. 1996;137(5):1545–1553. doi: 10.1210/endo.137.5.8612484. [DOI] [PubMed] [Google Scholar]
- 25.Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138(12):5398–5407. doi: 10.1210/endo.138.12.5624. [DOI] [PubMed] [Google Scholar]
- 26.Plotkin LI, Bellido T. Beyond gap junctions: connexin 43 and bone cell signaling. Bone. 2013;52(1):157–166. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong D, Lu X, Wang HX, et al. A dominant loss-of-function GJA1 (Cx43) mutant impairs parturition in the mouse. Biol Reprod. 2009;80(6):1099–1106. doi: 10.1095/biolreprod.108.071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unlugedik E, Alfaidy N, Holloway A, et al. Expression and regulation of prostaglandin receptors in the human placenta and fetal membranes at term and preterm. Reprod Fertil Dev. 2010;22(5):796–807. doi: 10.1071/RD09148. [DOI] [PubMed] [Google Scholar]
- 29.Wang QH, Teng KD, Liu FH, et al. The effects of Yimu San on labor capacity of pregnant swine. Heilongjiang Anim Sci Vet Med. 2016;2016(11):176–178. 176. doi: 10.13881/j.cnki.hljxmsy.2016.2121. (in Chinese) [DOI] [Google Scholar]
- 30.Zhou BH, Liu RX, Jiang GJ, et al. Effects of ‘Yimu shenghua san’ on changes of placental hormones in retained placenta cows. Chin J Vet Sci. 2010;30(7):988–991. doi: 10.16303/j.cnki.1005-4545.2010.07.010. (in Chinese) [DOI] [Google Scholar]



