Abstract
This study aimed to evaluate fertility and oncologic outcomes in women with complex hyperplasia (CH) or complex atypical hyperplasia (CAH) who received fertility-sparing therapy and in vitro fertilization (IVF). Endometrial carcinoma is the most common carcinoma of the female genital tract, and is associated with endometrial hyperplasia (EH) resulting from long-term unopposed estrogenic stimulation of the endometrium. EH is characterized by non-physiological proliferation of endometrium that results in glands with irregular shapes and varying sizes. The World Health Organization (WHO) classified it into four types: simple or complex hyperplasia with or without atypia. CH is characterized by glands with irregular outlines that demonstrate marked structural complexity and back-to-back crowding. Atypical hyperplasia designates a proliferation of glands exhibiting cytologic atypia, in which varying degrees of nuclear atypia and loss of polarity are present. It has been reported that high-dose progestin is safe and efficient for CAH or early-stage low-grade carcinoma for young women who desire fertility-preserving treatment. However, few studies have reported the differences of pregnancy outcomes between patients with CAH and CH, while those patients take a great proportion in people suffered from infertility. More studies about the outcome of IVF are needed. Our aim is to evaluate fertility and oncological outcomes in women with CH or CAH who received fertility-sparing therapy.
Keywords: Progestin therapy, Endometrial hyperplasia, In vitro fertilization
This study aimed to evaluate fertility and oncologic outcomes in women with complex hyperplasia (CH) or complex atypical hyperplasia (CAH) who received fertility-sparing therapy and in vitro fertilization (IVF). Endometrial carcinoma is the most common carcinoma of the female genital tract (Stubert and Gerber, 2016), and is associated with endometrial hyperplasia (EH) resulting from long-term unopposed estrogenic stimulation of the endometrium. EH is characterized by non-physiological proliferation of endometrium that results in glands with irregular shapes and varying sizes (Horn et al., 2007). The World Health Organization (WHO) classified it into four types: simple or complex hyperplasia with or without atypia. CH is characterized by glands with irregular outlines that demonstrate marked structural complexity and back-to-back crowding. Atypical hyperplasia designates a proliferation of glands exhibiting cytologic atypia, in which varying degrees of nuclear atypia and loss of polarity are present (Kurman et al., 1985). It has been reported that high-dose progestin is safe and efficient for CAH or early-stage low-grade carcinoma for young women who desire fertility-preserving treatment (Randall and Kurman, 1997). However, few studies have reported the differences of pregnancy outcomes between patients with CAH and CH, while those patients take a great proportion in people suffered from infertility. More studies about the outcome of IVF are needed. Our aim is to evaluate fertility and oncological outcomes in women with CH or CAH who received fertility-sparing therapy.
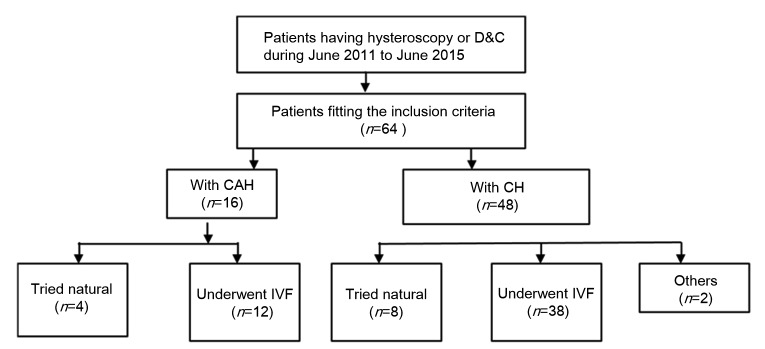
We conducted a retrospective analysis of patients who were ≤45 years old, diagnosed with either CAH or CH, who were treated with oral progestin for fertility preservation at the Center for Reproductive Medicine of Shandong Provincial Hospital Affiliated to Shandong University (Jinan, China) from June 2011 to June 2015. The follow-up evaluations were completed in June 2016. The follow-up time was between 1 and 5 years. A chart showing the group division is shown in Fig. 1. Diagnosis and responses to treatment were based on hysteroscopy surgery or dilation and curettage (D&C). Patients were categorized into either the CAH group or the CH group. Patients underwent adequate radiological examination before conservative management, and body mass index (BMI) was calculated at the beginning of treatment. The patients who chose medical therapy were counseled extensively regarding the risk of recurrence or progression. All included patients wished to preserve the possibility of pregnancy. Patients were included if they met the following five inclusion criteria: (1) pathologically confirmed CAH or CH after hysteroscopy; (2) disease confined to the endometrium; (3) absence of myometrial invasion and extrauterine spread based on diagnostic imaging; (4) infertility; (5) IVF was performed in our hospital. Progestin regimen was 250 mg of medroxyprogesterone acetate (MPA) daily for both CAH and CH patients. The patients underwent multiple biopsies (every 12 weeks) while receiving progestin therapy. Hysterectomy was performed when recurrence or progression appeared and patients refused to continue conservative management. IVF was proposed to couples and those who agreed underwent the procedure in our institute. Clinical and demographic data were obtained from medical records. If fertility data were insufficient, additional information was obtained by telephone interview.
Fig. 1.
Flow chart of data collection and group division
In our study, the 64 patients who met the inclusion criteria were treated with progestin and all had pregnancy attention. According to the pathologic analysis, 48 patients had CH and 16 had CAH. Table 1 displays the baseline characteristics of all patients included in our study. There were no significant demographic differences between the CAH and CH groups. The mean age was 32.5 years in the CAH group and 32.7 years in the CH group. Thirty-nine patients were diagnosed with primary infertility and the other 25 patients were diagnosed with secondary infertility. A very large proportion of patients had irregular menstruation; 17 had an endometrial polyp and 4 patients had intrauterine adhesions. Treatment included hysterectomy, D&C, and oral progesterone.
Table 1.
Baseline characteristics of the study population
| Group | Age at diagnosis (year) | Infertility type |
BMI (kg/m2) | Abnormal uterine bleeding | Diabetes mellitus type II | Polycystic ovary syndrome | Thyroid disorder | Pituitary diseases | Tubal disease | Non-responsive | |
| Primary infertility | Secondary infertility | ||||||||||
| CAH (n=16) | 32.5±4.6 | 13 (81.25%) | 3 (18.75%) | 26.7±5.2 | 8 (50%) | 2 (12.5%) | 2 (12.5%) | 0 | 0 | 2 | 1 |
| CH (n=48) | 32.7±4.6 | 26 (54.17%) | 22 (45.83%) | 24.8±3.7 | 26 (40.6%) | 2 (4.17%) | 9 (18.75%) | 4 (8.33%) | 1 (2.08%) | 1 | 0 |
|
| |||||||||||
| P-value | 0.846 | 0.054 | 0.137 | 0.772 | 0.223 | 0.566 | 0.564 | 1.000 | 0.088 | 0.250 | |
BMI: body mass index. Data are expressed as number, number (percentage), or mean±standard deviation (SD)
The oncology outcomes were: only one patient received hysterectomy because of the persistence of CAH and no case of endometrial cancer was observed. Patients of CAH (93%, 15/16) and CH (100%, 48/48) were responsive to progesterone therapy. The response rate did not differ between the CAH and CH groups. The responsive definition is given in our previous paper (Li et al., 2016). The mean remission time was about half a year (data not shown because of the spread of the distribution).
Table 1 showed the main pregnancy outcomes of the two groups. By the end of follow-up, 25 of 64 patients (39.1%) achieved clinical pregnancies. Among these patients, 4 patients conceived naturally and others became pregnant with the help of IVF. Among the couples who sought IVF to help achieve pregnancy, 12 women had CAH and 38 had CH. Three cancelled embryo transfer. The clinical pregnancy rate for IVF was 50.0% (50.0% in the CH group vs. 50.0% in the CAH group; P=1.000). The live birth rate following IVF was 38.0% (41.0% in the CH group vs. 25.0% in the CAH group; P=0.497). Notably, one patient who failed to achieve pregnancy following embryo transplantation conceived later without medical intervention. Based on our analysis of the outcomes of IVF, including fresh and frozen cycles, the cumulative live birth rate did not differ significantly between the two groups, including 3 patients in the CAH group and 16 patients in the CH group.
MPA is the most commonly used agent to treat CAH and CH, but megestrol acetate and levonorgestrel-releasing intrauterine system have both been reported to be effective (Gadducci et al., 2009; Orbo et al., 2014).
It is uncertain whether the outcomes and feasibility of fertility-sparing treatment differ between those women with CAH and CH. A report demonstrated that 74% (39/53) patients with CAH/grade I endometrial cancer achieved complete response after a median period of 6 (3–24) months (Chen et al., 2016). We cannot ignore the risk of relapse with conservative treatment. A meta-analysis revealed that a BMI of 30 kg/m2 or higher is strongly associated with relapse of CAH after initial regression after conservative treatment (Yang et al., 2015). Another study reported that relapse occurred less often for women with CH (28.3%; 95% confidence interval (CI) 18.5%–40.8%; 17/60 for oral progestogens) than with CAH (50%; 95% CI 18.7%–81.2%; 3/6 for oral progestogens), and pointed out that a BMI of 35 kg/m2 or higher is strongly associated with relapse of CAH after initial regression (Gallos et al., 2013).
In our study, we compared the reproductive outcomes of the two groups and found no differences between them. Inoue et al. (2016) identified three factors considered to affect pregnancy establishment following conservative treatment with MPA: recurrence, endometrial thickness during ovulation, and the age of the pregnancy permission. The common factors associated with IVF outcomes are listed in Table S1, including infertility type, BMI, etc. The CAH and CH groups did not differ significantly in these risk factors. The other debate is on whether ovarian stimulation during IVF affects disease progression. A meta-analysis revealed that IVF does not seem to be associated with elevated cervical cancer risk, nor with ovarian or endometrial cancer (Siristatidis et al., 2013).
Our research suggests that oral progestin is a useful temporizing treatment and IVF is effective. Introduction of IVF soon after achieving tumor disappearance by MPA would therefore be beneficial for patients with disease recurrence, thin endometrium, or a higher age of pregnancy permission (Inoue et al., 2016). The implementation of IVF techniques not only increases the chance of conception, but it may also decrease the interval to conception (Gadducci et al., 2009). A multivariate analysis suggested that patients who accepted assisted reproductive technology (ART) were more likely to become pregnant (Zhou et al., 2015). A recent meta-analysis evaluated the live birth rate after fertility-sparing management and showed that the live birth rate after ART (39.4%) was significantly higher than that after spontaneous pregnancy (14.9%) (Gallos et al., 2013). However, some can still become pregnant naturally, but may wish to achieve pregnancy as soon as possible. Moreover, the patients underwent repeated D&C which may confer harm to the endometrial thickness and receptivity and thereby negatively impact the success of IVF. For patients with a partner, the best option is IVF. On the one hand, for some patients who suffered from polycystic ovary syndrome (PCOS) or other diseases, IVF is a good option to improve the likelihood of pregnancy. Choosing IVF strategy as soon as possible gives patients the chance to accept a radical operation.
Contributors
Lei YAN participated in the design of the study. Miao LI and Jia-lun SONG analyzed the data and drafted the manuscript. Ying ZHAO, She-ling WU, and Hong-bin LIU did the follow-up. Rong TANG participated in discussion of the results and helped revise the manuscript.
Acknowledgments
We thank BioMed Proofreading LLC for revising the manuscript. We thank Prof. Zi-jiang CHEN of Shandong University (Jinan, China) for her kind guidance of the study.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81571414), the key Research and Development Program of Shandong Province (No. 2015gsf118124), and the Chinese Medical Association of Clinical Medicine Research Special Funds (No. 16020290645), China
Compliance with ethics guidelines: Miao LI, Jia-lun SONG, Ying ZHAO, She-ling WU, Hong-bin LIU, Rong TANG, and Lei YAN declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for whom identifying information is included in this study.
References
- 1.Chen M, Jin Y, Li Y, et al. Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int J Gynaecol Obstet. 2016;132(1):34–38. doi: 10.1016/j.ijgo.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 2.Gadducci A, Spirito N, Baroni E, et al. The fertility-sparing treatment in patients with endometrial atypical hyperplasia and early endometrial cancer: a debated therapeutic option. Gynecol Endocrinol. 2009;25(10):683–691. doi: 10.1080/09513590902733733. [DOI] [PubMed] [Google Scholar]
- 3.Gallos ID, Ganesan R, Gupta JK. Prediction of regression and relapse of endometrial hyperplasia with conservative therapy. Obstet Gynecol. 2013;121(6):1165–1171. doi: 10.1097/aog.0b013e31828cb563. [DOI] [PubMed] [Google Scholar]
- 4.Horn LC, Meinel A, Handzel R, et al. Histopathology of endometrial hyperplasia and endometrial carcinoma: an update. Ann Diagn Pathol. 2007;11(4):297–311. doi: 10.1016/j.anndiagpath.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Inoue O, Hamatani T, Susumu N, et al. Factors affecting pregnancy outcomes in young women treated with fertility-preserving therapy for well-differentiated endometrial cancer or atypical endometrial hyperplasia. Reprod Biol Endocrinol. 2016;14:2. doi: 10.1186/s12958-015-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia: a long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56(2):403–412. doi: 10.1002/1097-0142(19850715)56:2%3C403::aid-cncr2820560233%3E3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Bai Y, Yan L, et al. SPAG9 may be a potential prognostic markerof endometrial hyperplasia and grade 1 endometrioid adenocarcinoma treated with progestin. Gynecol Obstet Invest. 2016;81(3):267–274. doi: 10.1159/000437015. [DOI] [PubMed] [Google Scholar]
- 8.Orbo A, Vereide A, Arnes M, et al. 2014. [Google Scholar]
- 9.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90(3):434–440. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 10.Siristatidis C, Sergentanis TN, Kanavidis P, et al. Controlled ovarian hyperstimulation for IVF: impact on ovarian, endometrial and cervical cancer–a systematic review and meta-analysis. Hum Reprod Update. 2013;19(2):105–123. doi: 10.1093/humupd/dms051. [DOI] [PubMed] [Google Scholar]
- 11.Stubert J, Gerber B. Current issues in the diagnosis and treatment of endometrial carcinoma. Geburtsh Frauenheilk. 2016;76(2):170–175. doi: 10.1055/s-0035-1558230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang YF, Liao YY, Liu XL, et al. Prognosticfactors of regression and relapse of complex atypical hyperplasia and well-differentiated endometrioid carcinoma with conservative treatment. Gynecol Oncol. 2015;139(3):419–423. doi: 10.1016/j.ygyno.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Zhou R, Yang Y, Lu Q, et al. Prognostic factors of oncological and reproductive outcomes in fertility-sparing treatment of complex atypical hyperplasia and low-grade endometrial cancer using oral progestin in Chinese patients. Gynecol Oncol. 2015;139(3):424–428. doi: 10.1016/j.ygyno.2015.09.078. [DOI] [PubMed] [Google Scholar]