Table 3.
Scope of different nitrones
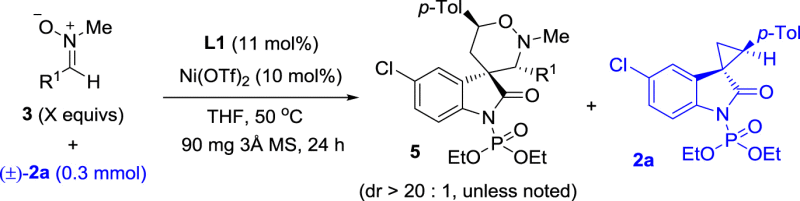
| |||||
|---|---|---|---|---|---|
| Entry | Nitrone 3 | X | 5: Y/ee(%)a, b | 2a: R/ee(%)b, c | s d selectivity |
| 1 | 3a: R1 = Ph | 0.55 | 5a: 42/96 | 45/97 | 36 |
| 2 | 3b: R1 = p-Tolyl | 0.56 | 5b: 45/98 | 48/97 | 76 |
| 3 | 3c: R1 = p-MeOC6H4 | 0.55 | 5c: 37/96 | 42/99 | 31 |
| 4 | 3d: R1 = p-ClC6H4 | 0.55 | 5d: 41/97 | 41/97 | 21 |
| 5 | 3e: R1 = p-BrC6H4 | 0.55 | 5e: 40/97 | 50/92 | 79 |
| 6 | 3f: R1 = p-FC6H4 | 0.57 | 5f: 45/95 | 47/99 | 80 |
| 7 | 3g: R1 = m-MeOC6H4 | 0.55 | 5g: 46/95 | 49/90 | 42 |
| 8 | 3h: R1 = 2-naphthyl | 0.56 | 5h: 42/97 | 48/98 | 91 |
| 9 | 3i: R1 = 2-furyl | 0.56 | 5i: 48/98 | 44/99 | 41 |
| 10 | 3j: R1 = 2-thienyl | 0.56 | 5j: 42/97 | 48/90 | 33 |
| 11 | 3k: R1 = i-Pr | 0.57 | 5k: 42/93 | 40/77 | 7 |
| 12e | 3l: R1 = Cy | 0.56 | 5l: 36/90 | 45/79 | 11 |
aY/ee: isolated yield and ee value of 5
bEe determined by chiral HPLC analysis
cR/ee: the recovery and ee value of 2a
d s = ln[(1 − C)(1 − ee)]/ln[(1 − C)(1 + ee)]; C refers to the conversion of (±)−2a (1-(yield of recovered 2a))
e13:1 dr
