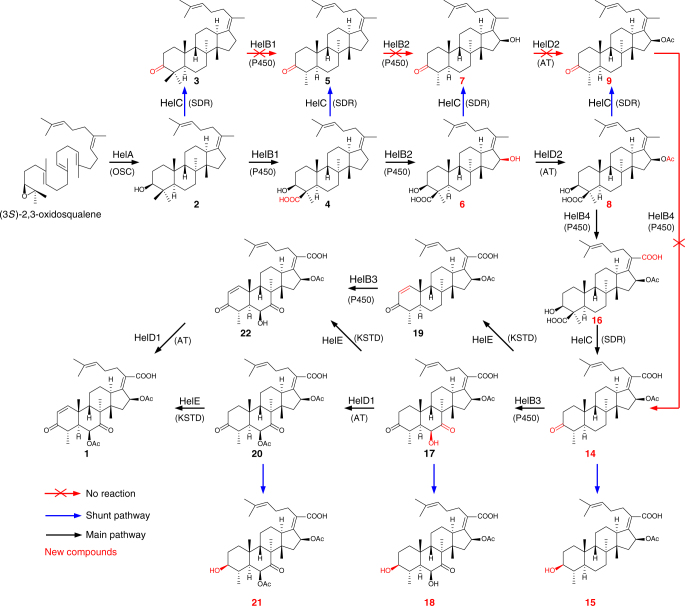
Fig. 4.
Complete biosynthetic pathway of helvolic acid. Biosynthesis of helvolic acid includes nine steps from the universal triterpenoid progenitor (3 S)-2,3-oxidosqualene. Following the cyclization to the tetracyclic protosta-17(20)Z,24-dien-3β-ol (2) by HelA, HelB1-mediated and HelB2-mediated oxidation at C-4 and C-16, HelD2-dependent acetylation of 16-OH, oxidation of C-21 by HelB4, and HelC-dependent oxidative decarboxylation yield the fusidane skeleton 14, which is further modified in three additional steps mediated by HelB3, HelD1, and HelE to give helvolic acid. The premature decarboxylation (or dehydrogenation) by HelC prevents HelB1-mediated, HelB2-mediated, HelD2-mediated, and HelB4-mediated tailoring