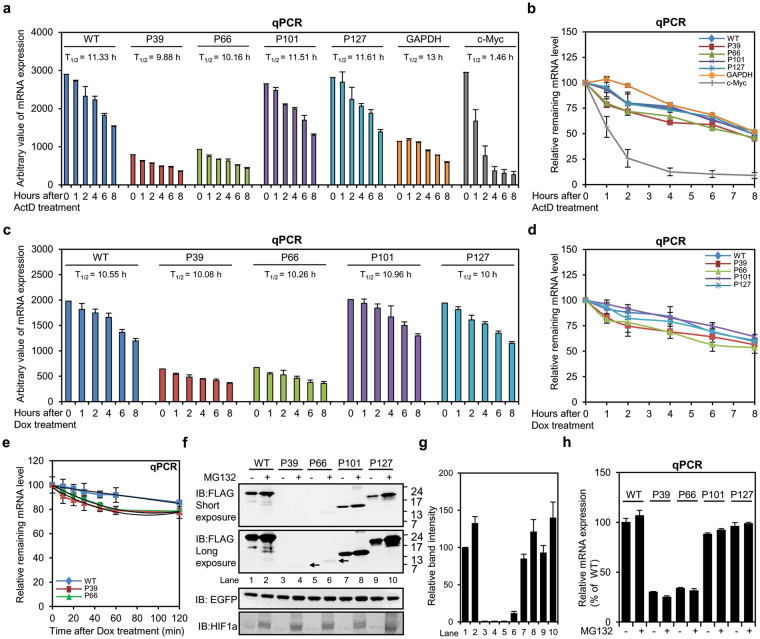
Figure 2.
The undegraded PTC-containing mRNAs from gBglo-P39 and gBglo-P66 are highly stable, but generate trace amounts of mutant proteins in a steady state. (a,b) qPCR analysis of mRNA expressed from expression vectors gBglo-WT, gBglo-P39, gBglo-P66, gBglo-P101, and gBglo-P127 in HeLa cells at indicated times after treatment with Actinomycin D (ActD). GAPDH and c-Myc served as stable and highly unstable controls, respectively. (c,d) qPCR analysis of mRNA expressed from Tet-Off β-globin expression vectors gBglo-WT, gBglo-P39, gBglo-P66, gBglo-P101, and gBglo-P127 in HeLa cells at indicated times after treatment with Doxycycline (Dox). Arbitrary values and relative values are shown in (a) and (c), and in (b) and (d), respectively. (e) qPCR analysis of mRNA expressed from gBglo-WT, gBglo-P39, and gBglo-P66 at early time points after Dox treatment. (f) Western analysis of wild-type and mutant protein expression from the indicated genomic β-globin constructs in HeLa cells with or without proteasome inhibitor MG132. Arrows indicated the expected positions of mutant proteins. GAPDH served as internal control. HIF1α expression was measured to confirm inhibition of proteasomal degradation by MG132. (g) Quantification of band intensities for the western blot shown in (f). (h) qPCR analysis of mRNA expressed from the indicated genomic β-globin constructs in HeLa cells with or without MG132. Every experiment summarized in Fig. 2 was performed two independent times and the results of one representative experiment are shown. Error bars in (a–e) and (h), and in (g) represent the SD of the mean of two independently performed qPCR analyses and the SD of the mean of band intensities obtained from two independently performed western blots, respectively. IB, immunoblot.