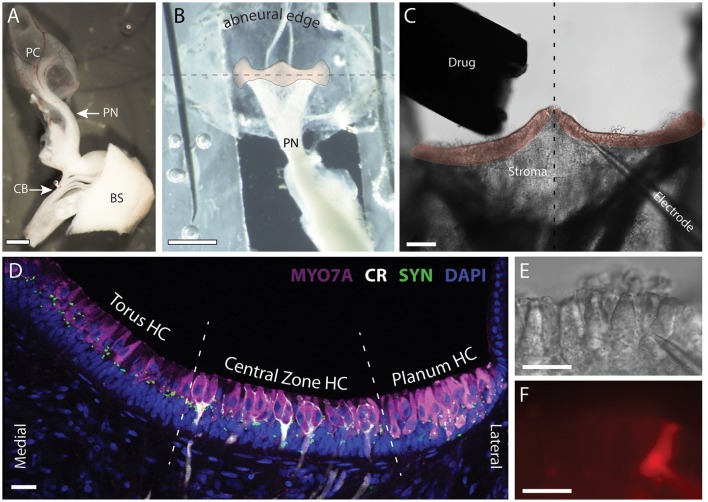
Figure 1.
Patch clamp recordings were made from intact hair cells in a split-epithelial preparation. (A) The posterior canal (PC), the posterior nerve (PN), and its connectivity to the posterior division of cranial nerve VIII are microdissected from the turtle inner ear. A small section of the brainstem (BS), anterior trunk of CNVIII, and the efferent crossbridge (CB), are also preserved. (B) The roof of the posterior canal ampulla is cut, splayed, and immobilized with minutien pins to expose a bird's eye view of the crista neuroepithelium (orange overlay). The abneural edge of the ampulla is folded along the longitudinal torus-to-planum axis of the crista (dashed line) and wrapped underneath to rotate the crista forward into the bottom of the recording dish. (C) The upward facing stroma and neuroepithelium were removed by ripping the tissue to expose the underlying hair cells and afferents for subsequent patch-clamp recordings. The drug delivery pipette and nearby patch electrode are also shown. Dashed line bisects the torus to reveal the left and right hemicristae (orange overlay). (D) Confocal image projection of a longitudinal section of a turtle hemicrista where hair cells from the torus, central zone (CZ), and planum regions were stained with antibodies to myosin 7A (MYO7A, magenta). Anti-calretinin (CR, white) labeled calyx-bearing afferents in the central zone and type II hair cells in the planum. Synapsin antibodies (SYN, green) labeled vestibular efferent terminals across the neuroepithelium. Orientation of section corresponds to the right hemicrista in (C). Dashed lines delineate the approximate boundaries of torus, central zone, and planum regions from which hair cells (HC) were recorded. (E,F) DIC and fluorescent image of patch pipette and type II hair cell near the torus, respectively. Alexa594 sodium hydrazide (50 μM) was included in the patch pipette. Scale Bars (in μm): (A,B) = 500; (C) = 100; (D–F) = 20.