Table 1.
List of clinical trials evaluating OVs in solid tumours
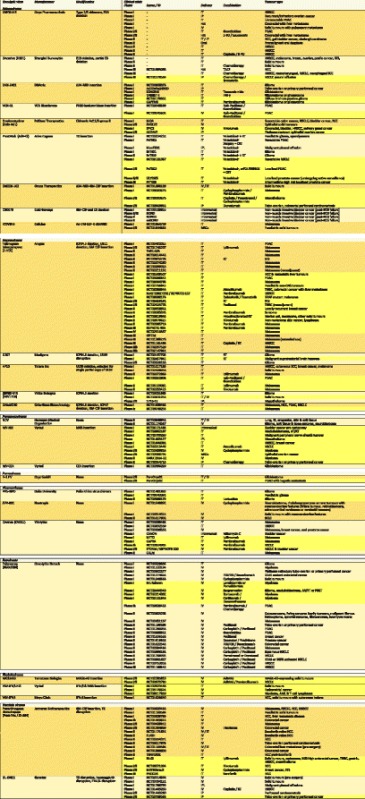
Table updated and amended from [13]. Clinical trials highlighted in yellow are currently recruiting or not yet recruiting patients as of 28th August 2017 on clinicaltrials.gov. AdMA3 Adenovirus with transgenic MAGE-A3 insertion, AML acute myeloid leukaemia, AT/RT atypical teratoid rhabdoid tumour, BCG Bacillus Calmette-Guérin, CEA carcinoembryonic antigen, CNS central nervous system, CRT chemoradiotherapy, EGFR epidermal growth factor receptor, GM-CSF granulocyte–macrophage colony-stimulating factor, HAI hepatic arterial infusion, HCC hepatocellular carcinoma, hNIS human sodium iodide symporter, HNSCC head and neck squamous cell carcinoma, IFN-β interferon beta, IP intraperitoneal, IPL intrapleural, IT intratumoural, IV intravenous, MAGE-A3, melanoma associated antigen 3, MSC mesenchymal stem cells, MSI microsatellite instability, MV measles virus, NDV Newcastle disease virus, NSCLC non-small-cell lung cancer, PDAC pancreatic ductal adenocarcinoma, PNET primitive neuroectodermal tumour, RCC renal cell carcinoma, RGD Arg-Gly-Asp motif, RT radiotherapy, SCC squamous cell carcinoma, SCLC small cell lung cancer, STS soft tissue sarcoma, SVV, Seneca Valley virus, TACE transarterial chemoembolization, TK thymidine kinase, TNBC triple negative breast cancer, US11 unique short 11 glycoprotein, VSV vesicular stomatitis virus
