Abstract
A 56-year-old man developed mid-gut bowel ischaemia following an elective aortobiprofunda bypass for short-distance claudication. The bowel was resected and he was commenced on lifelong total parenteral nutrition. He was found to have developed heparin-induced thrombocytopenia and thrombosis, confirmed by high levels of heparin-platelet factor 4-antibody on enzyme-linked immunosorbent assay (ELISA). He subsequently had foregut ischaemia with a second bout of thrombocytopenia despite not being on heparin. The case describes the first report of bowel ischaemia as a consequence of heparin-induced thrombocytopenia causing sequential superior mesenteric and coeliac arterial thrombosis in this scenario and highlights the importance of the awareness of the association of these pathological entities and subsequent management.
Keywords: Aortobiprofunda, Heparin-induced thrombocytopenia, Bowel ischaemia
Case history
A 56-year-old smoker with short-distance claudication due to aorto-iliac and bilateral superficial femoral artery (SFA) occlusive disease underwent an elective aortobiprofunda bypass using a 16 × 8 mm Dacron prosthesis. His past medical history included an extended right hemicolectomy for dysplastic adenoma 10 years previously, at which time he received 5000 units unfractionated heparin (Minihep; Leo Laboratories Ltd, Longwick Road, Princes Risborough, Buckinghamshire, HP27 9RR, UK) subcutaneously, twice a day for 7 days as deep vein thrombosis (DVT) prophylaxis. Pre-operative computed tomographic (CT) angiography had shown mild ostial stenoses of the coeliac axis and superior mesenteric artery (SMA). He received 5000 units of unfractionated heparin IV during the procedure. Clamps were applied below the level of the renal arteries. Postoperatively, he had bilaterally warm peripheries. He was started on enoxaparin 40 mg once a day for DVT prophylaxis.
The patient was well until Day 2, when he developed vomiting and a nasogastric tube was inserted, resulting in a daily aspirate just exceeding a litre. Abdominal radiography at this time showed distended small bowel, and a subsequent CT scan showed small bowel dilatation with a mild calibre change corresponding to the previous ileotransverse anastomosis. The patient underwent a laparotomy on Day 8, at which a non-viable jejunum and ileum was found, with stripes of necrosis. No pulsation was detected in the SMA or its branches. No internal hernias were noted. The jejunum and ileum were resected in their entirety and an end duodenostomy and a sigmoid colon mucous fistula created, given the patient’s age, and the availability of in-hospital expertise in dealing with short bowel syndrome. Histology was not available.
Postoperatively, total parenteral nutrition was commenced. The platelet count reached 28 × 109/l on Day 9 (from initial operation), at which time a diagnosis of heparin-induced thrombocytopenia (HIT) was considered and a haematology opinion was obtained. At this point, there was a pre-test four T’s score of 7, indicating a high probability of HIT. Enoxaparin was stopped and danaparoid commenced at 750 units subcutaneously, twice a day. Heparin-platelet factor (PF) 4-antibody testing, from a sample taken on Day 9, using ELISA yielded an optical density of 3.064 (positive reactions classified as > 0.400), indicating a diagnosis of HIT type II on Day 17. At this time, the platelet count had recovered to 229 × 109/l. These trends are highlighted in Figure 1.
Figure 1.
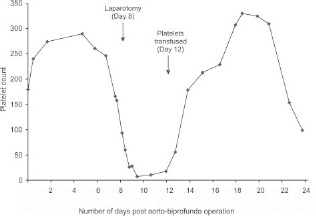
Postoperative platelet counts (× 109/l).
The patient initially improved; however, on Day 23, his platelet count had again dropped, reaching 154 × 109/l. Rapid deterioration thereafter necessitated a second laparotomy, at which time foregut necrosis and liver infarction were noted. He died later that day.
Discussion
Heparin-induced thrombocytopenia is a rare syndrome comprising a decline in platelet count following heparin administration. There are two subtypes of HIT. Type I is non-immune, usually resolving spontaneously within days, causing no significant clinical problems. Type II is an immune-mediated prothrombic disorder caused by predominantly IgG antibodies against heparin–PF4 complexes resulting in platelet activation, release of procoagulant protein kinases, thrombocytopenia, and excessive thrombin generation leading to venous or arterial thrombosis,1 with mortality up to 20–30%.2 The term ‘HIT’ from this point on in this paper refers to HIT type II. HIT is reported to develop in up to 3% of patients on unfractionated heparin and 0.1% of those on low molecular weight heparin (LMWH) depending on the clinical scenario. The presence of anti-PF4/heparin antibody is essential in making a diagnosis of HIT, although the presence of antibodies alone does not necessarily confirm HIT. Consequently, a pre-test probability score has been proposed (Table 1), where this score is taken in conjunction with the presence of antibodies to judge the likelihood of HIT. In this case study there was a pre-test score of 7 (at the time of testing on Day 9: the platelet count was 28 × 109/l, the platelet decline had started on Day 8, a thrombosis was evident, however, the possibility of other causes could not be excluded), indicating a high pre-test probability. This score when taken in conjunction with the presence of antibodies provides strong evidence for HIT.
Table 1.
Pre-test probability of HIT: ‘the four T’s’3
| Category | Score | ||
|---|---|---|---|
| 2 | 1 | 0 | |
| Thrombocytopenia | 50% fall or platelet nadir 20–100 × 109/l | 30–50% fall or platelet nadir 10–19 × 109/l | Fall < 30% or platelet nadir < 10 × 109/l |
| Timing* of platelet count fall or other sequelae | Clear onset between Days 5–10; or less than 1 day (if heparin exposure within past 100 days) ) | Consistent with immunisation but not clear (e.g. missing platelet counts or onset of thrombocytopenia after day 10 | Platelet count fall too early (without recent heparin exposure) |
| Thrombosis or other sequelae (e.g. skin lesions) | New thrombosis; skin necrosis; post-heparin bolus acute systemic reaction | Progressive or recurrent thrombosis; erythematous skin lesions; suspected thrombosis not yet proven | None |
| Other causes for thrombocytopenia not evident | No other cause for platelet count fall is evident | Possible other cause is evident | Definite other cause is present |
Pre-test probability score: 6–8, high; 4–5, intermediate; 0–3, low.
First day of immunising heparin exposure considered Day 0; the day the platelet count begins to fall is considered the day of onset of thrombocytopenia (it generally takes 1–3 days more until an arbitrary threshold that defines thrombocytopenia is passed).
There is no diagnostic test for HIT, although the serotonin-release assay (SRA) is the most specific. It works by exploiting the characteristic platelet-activating properties of HIT antibodies. The assay is technically demanding and consequently not used routinely in clinical practice. ELISA, which tests for IgG, IgA and IgM and not platelet activating antibodies, is the most reliable albeit not the most specific, as antibodies are not always associated with thrombocytopenia or even thrombosis. However, the antibody titre level in ELISA may help quantify the likelihood of HIT. Warkentin et al.4 recently showed that a heparin–PF4 antibody level of 2.00 or more equates to an approximately 90% risk of a strong positive serotonin-release assay (SRA). The ELISA in this case was 3.064, strongly suggesting a diagnosis of HIT.
Management of HIT comprises the discontinuation of heparin and commencement of an alternative at therapeutic levels, such as danaparoid (Orgaran Laboratories, Cambridge Science Park, Cambridge CB4 0FL, UK) or lepirudin (Pharmion Ltd, Berkshire SL4 1NA, UK). Of these, lepirudin seems to be the most efficacious in terms of best clinical outcomes. LMWH is contra-indicated due to cross-reactivity with PF4-heparin antibodies.
As far as the authors are aware, there has only been one previous report of bowel ischaemia as a result of HIT.5 In our case, we postulate that, following previous sensitisation 10 years ago, HIT developed following reexposure to heparin. The presence of a pre-existing stenosis at the SMA origin, together with the HIT-induced prothrombotic state, led to the formation of an ostial thrombus, with subsequent embolism into the distal branches, resulting in the infarcted stripes of bowel. Though there was a second drop in platelets on Day 23, it is not possible to determine whether this was a HIT-related phenomenon. There is, however, known cross-reactivity between danaparoid and HIT antibodies, and whether this is representative of a second prothrombotic insult remains speculative.
This report highlights the importance of a high index of clinical suspicion of HIT in any patient experiencing a drop in platelet counts postoperatively, and particularly in those with a background of SMA/coeliac ostial stenoses which may predispose to thrombotic ostial occlusion and gut ischaemia. We advocate the use of the ‘four Ts’ pre-test scoring system combined with the HIT assay in any situation where diagnostic uncertainty exists and there is more than one potential cause for thrombocytopenia.
References
- 1.Jang IK, Hursting MJ. When heparins promote thrombosis: review of heparin-induced thrombocytopenia. Circulation 2005; : 2671–83. [DOI] [PubMed] [Google Scholar]
- 2.Warkentin TE. Heparin-induced thrombocytopenia: a clinicopathologic syndrome. Thromb Haemost 1999; : 439–47. [PubMed] [Google Scholar]
- 3.Keeling D, Davidson S, Watson H. The management of heparin-induced thrombocytopenia. Br J Haematol 2006; : 259–69. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost 2008; : 1304–12. [DOI] [PubMed] [Google Scholar]
- 5.Buerger T, Tautenhahn J, Meyer F, Lippert H. Heparin-induced vascular occlusion in vasculosurgical patients. An evaluation of the disease in 13 cases. J Cardiovasc Surg (Torino) 1999; : 237–42. [PubMed] [Google Scholar]


