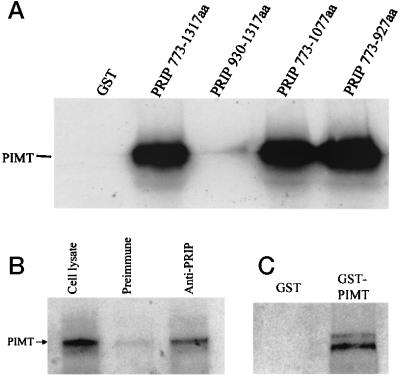
Figure 5.
In vitro interaction of PRIP with PIMT. (A) [35S]Methionine-labeled full-length PIMT generated by in vitro translation was incubated with glutathione-Sepharose beads bound with purified E. coli-expressed GST-PRIP (amino acids 773-1317, 930-1317, 773-1077, and 773–927) or GST. The bound proteins were eluted and analyzed by using 10% SDS/PAGE and autoradiographed. (B) Coimmunoprecipitation of PIMT and PRIP in intact cells. pcDNA3.1-FLAG-PIMT were transfected into COS-1 cells and, 24 h after transfection, cells were harvested. Cell lysate was immunoprecipitated with anti-PRIP or control serum, and immunoprecipitates were subjected to immunoblotting with anti-FLAG. Epitope-tagged protein PIMT can be coprecipitated by anti-PRIP (lane 3) but not by preimmune serum (lane 2). Lane 1, 1/20 input cell lysate. (C) PIMT forms homodimers. GST-PIMT (326-852 aa) or GST alone was incubated with PIMT labeled with [35S]methionine. The bound protein was electrophoresed and visualized by fluorography. GST-PIMT, but not GST alone, binds PIMT.