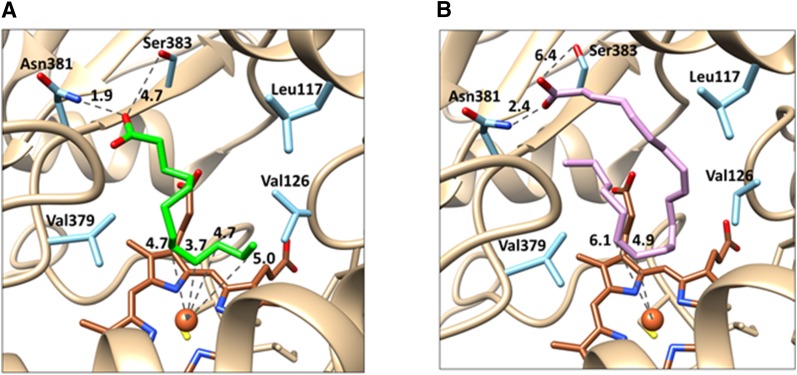
Fig. 6.
3D Homology models of the CYP4Z1 active site with LA and AA docked. The CYP4Z1 amino acid residues shown in light blue are believed to be of primary importance to substrate binding, with Leu117, Val126, and Val379 forming a hydrophobic pocket encapsulating the fatty chains of LA (green, A) and AA (lavender, B). Asn381 appears to anchor both substrates in the active site, likely through electrostatic interactions formed between the Asn amine and the fatty acid carboxylate moieties (∼2 Å distant). (A) The model of LA bound to CYP4Z1 shows internal carbons of the fatty acid centered over the heme (brown), with C-8 through C-11 of LA all located between 3.7 and 5.0 Å from the heme iron atom. By contrast, the terminal carbon of LA is oriented away from the heme iron at 6.3 Å. (B) The model of AA bound to CYP4Z1 likewise shows the terminal carbon of the fatty acid to be pointed away from the heme, whereas the C14-C15 double bond of AA is only 5 to 6 Å distant from the heme iron. In the figure, oxygen atoms are shown in red, nitrogen atoms in dark blue, sulfur atoms in yellow, and the heme iron as a brown sphere.