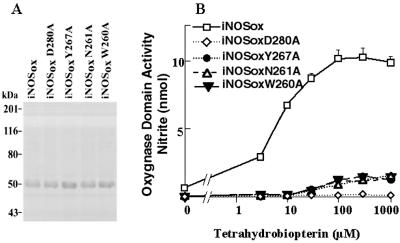
Figure 2.
Characterization of oxygenase domains of human iNOS and iNOS mutants. Using E. coli, the oxygenase domains (residues 71–504 plus a COOH-terminal His6 tag) of wild-type human iNOS (iNOSox) and iNOS mutants with Ala replacing Asp-280 (D280A), Tyr-267 (Y267A), Asn-261 (N261A), or Trp-260 (W260A) were generated and purified. (A) SDS/PAGE analysis confirmed the size and the purity of the recombinant proteins. (B) Oxygenase domain activity was assessed by measuring H2O2-supported oxidation of Nω-hydroxy-l-Arg. Protein samples were preincubated for 30 min with the indicated concentrations of H4B before initiating the reaction with H2O2. Nitrite values are the amount formed in 10 min at 37°C. Data are means ± SD of three experiments.