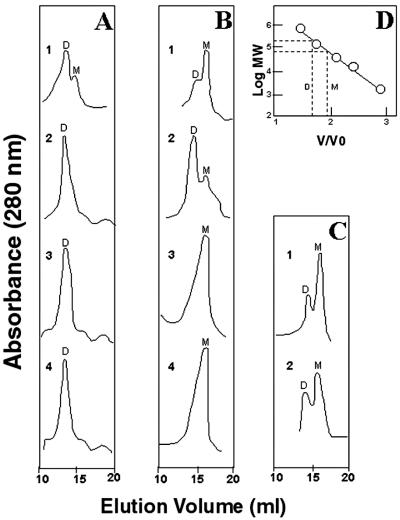
Figure 3.
Gel-permeation chromatography of oxygenase domains of wild-type human iNOS and iNOS mutants. (A) Human iNOSox and iNOSoxD280A were analyzed in the absence (traces 1 and 3, respectively) and in the presence (traces 2 and 4, respectively) of 5 mM l-Arg and 20 μM H4B. (B) Elution profiles of urea-generated monomers of iNOSox and iNOSoxD280A (traces 1 and 3, respectively) and of the urea monomers after incubation of iNOSox with 5 mM l-Arg and 20 μM H4B (trace 2) or of iNOSoxD280A with 50 mM l-Arg and 1 mM H4B (trace 4). (C) iNOSoxY267A was analyzed in the absence or the presence of 50 mM l-Arg and 1 mM H4B (traces 1 and 2, respectively). (D) Calibration with Mr standards. The estimated relative retention of the oxygenase domain dimer and monomer is indicated. Letters D and M denote dimer and monomer, respectively. Data represent two experiments.