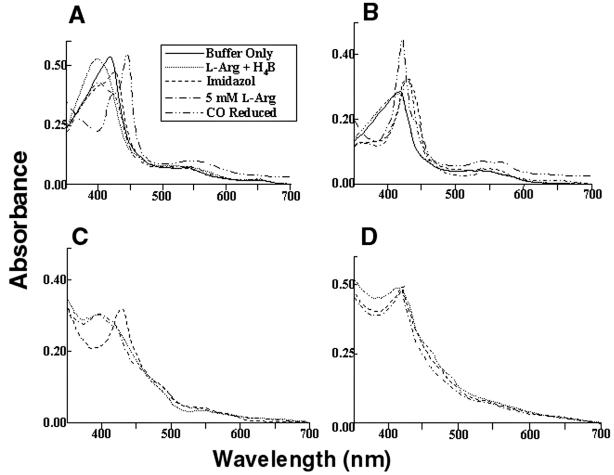
Figure 5.
Light absorbance spectral analysis of iNOSox (A), iNOSoxD280A (B), iNOS (C), and iNOSD280A (D). Inset represents line designation of each spectral trace for A–D, except that spectra with “buffer only” and after CO reduction pertain to A and B only. (A) Spectra of 7 μM iNOSox, recorded in 40 mM EPPS, pH 7.6, containing 5% glycerol (vol/vol) and 5 mM 2-mercaptoethanol at 28°C (−), had maximum absorbance at 417 nm, typical of low-spin hexa-coordinate heme protein. After incubation for 10 min at 28°C with 1 mM l-Arg and 20 μM H4B (… ), iNOSox spectra shifted toward high-spin penta-coordinate heme species, with maximum absorbance at 395 nm. Addition of 2 mM imidazole (—) converted heme to the low-spin state, with maximum absorbance at 427 nm. Addition of 5 mM l-Arg (-.-.-) to the imidazole-bound heme reverted its absorbance to 395 nm. CO binding reduced heme, shifting its absorbance to 444 nm. (B) Spectra of iNOSoxD280A showed the initial low-spin heme at 417 nm but did not convert to high spin with the addition of l-Arg and H4B. Although iNOSox280A could bind imidazole converting its heme absorbance to 427 nm but addition of excess l-Arg could not convert heme back to its high-spin state as seen in iNOSox. (C) Spectra of iNOS exhibiting behavior similar to iNOSox. (D) Spectra of iNOS D280A exhibiting behavior similar to iNOSoxD280A. Spectra are representative of two experiments.