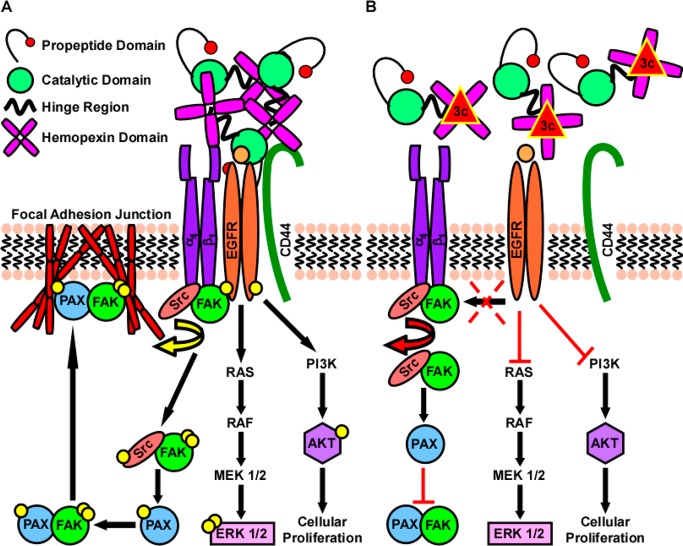
Figure 6.
Overview of the mechanism by which MMP-9 regulates formation of focal adhesion junctions. (A) During exocytosis and upon being secreted into the extracellular space, proMMP-9 forms a large complex with itself that can then act as a scaffold for promoting outside-in signaling. In our model, PEX-9 scaffolding promotes the association of β1 integrin-EGFR-CD44. Interaction with CD44 results in enhanced EGFR activation in addition to increased phosphorylation of its downstream targets AKT and Erk 1 + 2 (through the MAPK/Erk pathway). While complexed to β1 integrin, EGFR then goes on to transactivate Src kinase after it is recruited to the α4 integrin subunit during the generation of a new focal adhesion contact site. After activation, Src can then directly phosphorylate FAK currently associated with the β1 integrin subunit resulting in its maximal catalytic activity. This active Src–FAK complex can then bind and activate PAX, resulting in a mature FAK-PAX complex necessary for the formation of focal adhesion junctions. Formation of a complex between FAK and PAX results in final translocation to ECM–integrin junctions at the cell surface where they regulate cytoskeletal interactions resulting in enhanced cellular adhesion, migration, and invasion of cancer cells. (B) Treatment with PEX-9 inhibitor (depicted as red triangle) prevents MMP-9 scaffolding, thereby preventing downstream signaling driven by EGFR activation.