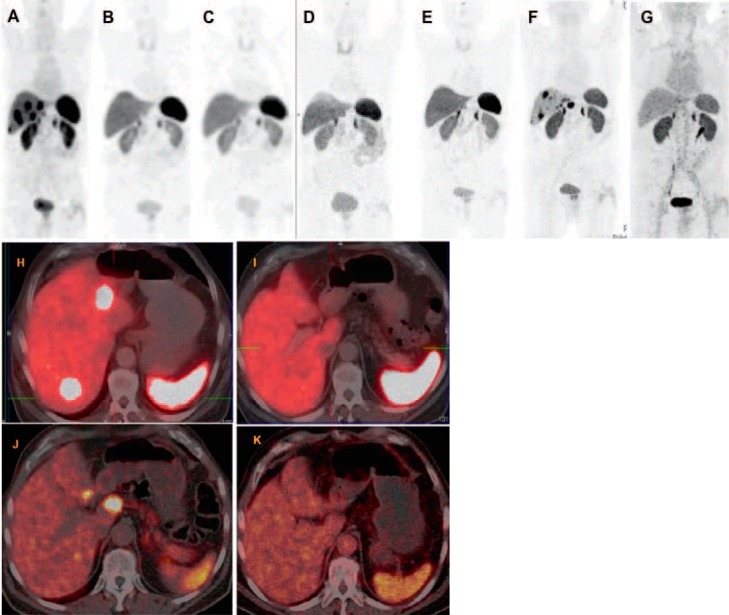
Fig. 1.
80-year-old male patient with grade I (Ki67 2%) well-differentiated, non-functioning neuroendocrine neoplasm at the rectosigmoid junction with extensive bilobar liver metastasis, diagnosed 6 years before, initial tumor stage cT3 pNx pM1 (HEP) stage IV, and immunohistochemical expression of chromogranin, synaptophysin, and somatostatin receptor (SSTR) IIa (in 60%). The first symptom at diagnosis was diarrhea with sonographic suspicion of liver metastases. Liver biopsy (performed at another hospital) demonstrated poorly differentiated adenocarcinoma; however, the primary tumor could not be detected by colonoscopy. Ga-68 SSTR positron emission tomography/computed tomography (PET/CT) identified an intensely SSTR-positive tumor at the rectosigmoid junction with multiple receptor-expressing hepatic metastases (maximum size 2 cm). There was no demonstrable glucose hypermetabolism in any of the tumor masses. Before referral to our center, the patient underwent systemic chemotherapy with FOLFOX and Avastin in addition to therapy with Sandostatin. There was progressive disease with continual increase in size of the liver metastases (to 3 and 3.4 cm 3 and 9 months after initiation of chemotherapy, respectively). Three cycles of Lu-177-based peptide receptor radionuclide therapy (PRRT) were administered (also based on a review of the histopathology which revealed neuroendocrine tumors), leading to a very good response with complete remission of the liver metastases and a significant decrease in the size (on CT) and SSTR expression (PET/CT) of the primary tumor. The patient was regularly followed up, i.e. every 9–12 months, with Ga-68 SSTR PET/CT. He had a progression-free interval of 4 years after which there were new SSTR-positive liver metastases. A second phase of PRRT (consisting of the 4th and 5th cycles) was administered, resulting again in an excellent response to PRRT with complete regression of the hepatic metastases. A–G PET maximum intensity projection images of Ga-68 SSTR PET/CT; H–K fused transverse PET/CT images; A, H before PRRT; B, K 4 months after the 3rd PRRT cycle; C–E 12, 24 and 36 months, respectively, after 3rd cycle of PRRT; F, J 48 months after the 3rd PRRT cycle a new hepatic progression was demonstrated; G, K once again complete remission of the liver metastases after a second phase of PRRT (4th and 5th cycles).