Abstract
Background
Lactate is measured in hospital settings to identify patients with sepsis and severe infections, and to guide initiation of early treatment. Point-of-care technology could facilitate measurement of lactate by clinicians in the community. However, there has been little research into its utility in these environments.
Aim
To investigate the effect of using point-of-care lactate at presentation to health care on mortality and other clinical outcomes, in patients presenting with acute infections.
Design and setting
Studies comparing the use of point-of-care lactate to usual care in initial patient assessment at presentation to health care were identified using a maximally sensitive search strategy of six electronic databases.
Method
Two independent authors screened 3063 records for eligibility, and extracted data from eligible studies. Quality assessment for observational studies was performed using the ROBINS-I tool.
Results
Eight studies were eligible for inclusion (3063 patients). Seven studies were recruited from emergency departments, and one from a pre-hospital aeromedical setting. Five studies demonstrated a trend towards reduced mortality with point-of-care lactate; three studies achieved statistical significance. One study demonstrated a significant reduction in length of hospital stay, although another did not find any significant difference. Two studies demonstrated a significant reduction in time to treatment for antibiotics and intravenous fluids.
Conclusion
This review identifies an evidence gap — there is no high-quality evidence to support the use of point-of-care lactate in community settings. There are no randomised controlled trials (RCTs) and no studies in primary care. RCT evidence from community settings is needed to evaluate this potentially beneficial diagnostic technology.
Keywords: general practice, lactate, point-of-care testing, pre-hospital care, primary health care, sepsis
INTRODUCTION
Sepsis is defined as the life-threatening organ dysfunction caused by a dysregulated host response to infection.1,2 Severe sepsis is thought to account for around 37 000 deaths annually in the UK3 — more than breast, bowel, and prostate cancer combined.4
Early recognition and treatment are key in preventing deaths from sepsis.2 Until recently, the focus has been on timely management in secondary care, with the introduction of sepsis care bundles and early warning scores.4,5 However, the latest National Institute for Health and Care Excellence (NICE) guidance recognises that systems need to extend to primary care, to facilitate timely recognition and prompt treatment.2 In addition, improving outcomes from sepsis has been highlighted as a clinical priority by the Royal College of General Practitioners6 and NHS England.7
Lactate measurement is frequently used in hospital settings to identify critical medical illness including sepsis and severe infections, and to guide treatment. With the recent increase in availability of point-of-care (POC) testing technology, possibilities for earlier biochemical testing in community settings have arisen.8
Community clinicians, the first point of assessment for many patients with sepsis, are in need of guidance regarding the added value of POC lactate in these settings. There are potential disadvantages of using such a test earlier in the pathway, such as increasing the time taken for assessment, false reassurance in emerging septic shock, or a much higher false positive rate in a setting of much lower prevalence, leading to inappropriate care escalation.
Accordingly, a systematic review was undertaken to evaluate whether the use of point-of-care lactate testing at first presentation to any healthcare setting in a population of adults and children with symptoms suggestive of serious bacterial infection reduces mortality or improves other clinical outcomes or markers of quality of care. These include time to antibiotics and length of any subsequent hospital stay, when compared with usual care.
METHOD
Search strategy
The authors searched MEDLINE (1946 to present), Embase (1974 to 3 June 2016), Web of Science (1945 to present), CENTRAL (issue 5 of 12, May 2016), Database of Abstracts of Reviews of Effects (issue 2 of 4, April 2015), and the Cochrane Database of Systematic Reviews (issue 6 of 12, June 2016) for relevant articles, using a maximally sensitive strategy (Appendix 1).
How this fits in
Sepsis accounts for around 37 000 deaths annually in the UK. Lactate is often measured in the hospital setting. The availability of point-of-care lactate allows measurement in community and pre- hospital environments such as primary care. Point-of-care lactate at presentation to healthcare setting may reduce mortality. The quality of evidence is low and no studies have previously been conducted in an out-of-hours or general practice setting.
The authors excluded animal studies, case reports, comments, letters, and editorials. All other study types were included in the search strategy. The authors searched for studies in both children and adults. There were no limits on language or date of publication. The authors performed citation searches of all full-text papers retrieved, to identify other relevant studies.
Data extraction
Following exclusion of duplicate studies, all titles and abstracts were screened independently by two authors, using the following inclusion criteria:
population — patients presenting to first-assessment settings, including community-based health care and emergency departments (EDs), with symptoms suggestive of serious bacterial infection;
intervention — point-of-care lactate testing;
comparator — usual care; and
outcomes — at least one patient outcome (for example, mortality, time to treatment, length of stay).
Purely diagnostic accuracy studies were excluded, given this review’s focus on clinical outcomes (and the potential circularity of lactate measurement being required for diagnosis of sepsis according to some existing definitions of sepsis). The full texts of remaining articles were independently screened by pairings of two authors, and reviewed for inclusion according to the specified criteria. Disagreements were resolved by discussion with a third reviewer.
Two authors independently extracted data using a proforma. The full papers were used where possible (four studies), with abstracts used if no full paper was available (four studies). The primary outcome was mortality. Secondary outcomes included time to lactate result, time to antibiotic and intravenous (IV) fluid treatment, and length of stay.
Authors were contacted for further clarification or missing data where necessary.
Quality assessment
Quality assessment was undertaken independently by two authors using the ROBINS-I tool for non-randomised studies of interventions.9 Quality was determined on a scale from low risk of bias (comparable with a well-performed randomised controlled trial [RCT]), to critical risk of bias (too problematic to provide any useful evidence on the effects of the intervention).
Data synthesis
There were insufficient data with acceptable risk of bias to perform meta-analysis.
RESULTS
Study characteristics
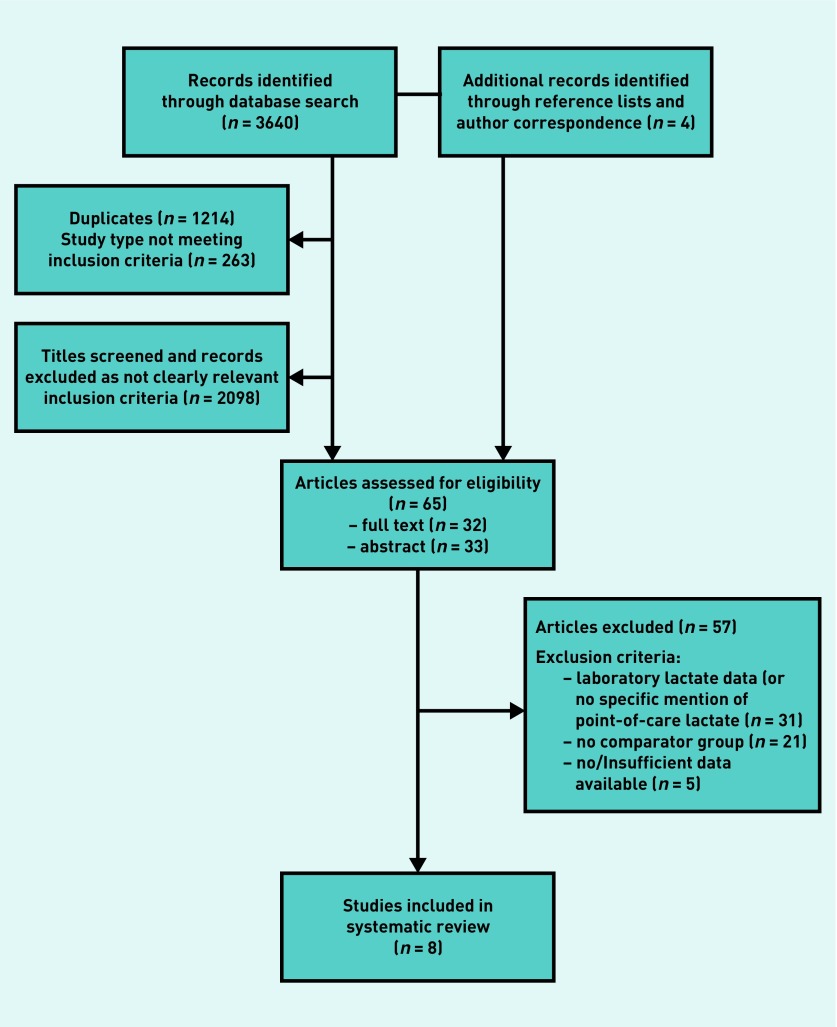
In all, 3644 titles and abstracts were screened, with 65 articles subsequently assessed for eligibility, of which 32 were full texts, and 33 records where abstracts only were available (Figure 1). Of these, 57 studies were excluded — 31 did not specify point-of-care lactate testing, 21 lacked a comparator group, and five provided insufficient data for inclusion. Eight studies (3063 patients) were included in the analysis (Table 1).
Figure 1.
Flowchart of included studies.
Table 1.
Characteristics of included studies
| Study | Study design | Setting | Intervention, n | Control, n | Lactate measurement sample/device | Outcome measures |
|---|---|---|---|---|---|---|
| Singer, 201510 | Before and after | Emergency department | 80 | 80 | Venous sample; portable i-STAT system (Abbott POC) | POCL measurement; resource utilisation; total hospital costs |
| Singer, 201411 | Before and after | Emergency department | 80 | 80 | Venous sample; portable i-STAT system (Abbott POC) | POCL measurement; time to lactate result; mortality; resource utilisation; IV fluids; antibiotics |
| Larsen, 201112 | Before, during, and after | Paediatric emergency department | 192 | 55 | Venous blood gas sample | POCL measurement; mortality; resource utilisation; sepsis bundle compliance; total hospital costs |
| Mullen, 201413 | Prospective observational cohort | Pre-hospital aeromedical | 20 | 39 | Fingerstick; lactate plus POC device (Nova Biomedical) | POCL measurement; mortality; IV fluids; transfusion; intubation; CVC line |
| Maung, 201414 | Observational cohort | Emergency department | 363 | 502 | Not specified | POCL measurement; mortality; IV fluids; antibiotics |
| Choong, 201415 | Observational cohort | Emergency department | 609 | 821 | Not specified | POCL measurement; mortality |
| Smith, 201016 | Observational cohort | Emergency department | 29 | 181 | Not specified | POCL measurement; time to lactate result; IV fluids; antibiotics; CVC line; sepsis bundle compliance |
| Varpula, 200717 | Sub-analysis; prospective observational cohort | Emergency department, intensive care unit | 53 | 39 | Arterial blood gas sample | POCL measurement; mortality; sepsis bundle compliance |
CVC = central venous catheter. IV = intravenous. POC = point of care. POCL = point-of-care lactate.
Three before-and-after10–12 and five observational cohort studies13–17 were included. In the cohort studies, there was limited description of the nature of allocation to point-of-care testing versus control. Seven studies recruited from emergency departments.10–12,14–17 Of these, four14–17 examined only a sub-population admitted from the ED to the intensive care unit (ICU) and, of these, two14–15 included only patients who were subsequently mechanically ventilated. One study had a pre-hospital setting,13 describing patients being transported by an aeromedical service. The studies — conducted in the US (five), Singapore (two), and Finland (one) — examined a total of 1346 point-of-care lactate results. Seven studies recruited from adult populations,10,11,13–17 and one from a paediatric population.12
In two studies, the primary focus was not on point-of-care lactate testing, but evaluation of sepsis treatment targets,17 and introduction of septic shock protocol.13 There were no studies undertaken in general practice settings, out-of-hours primary care, or ambulance services (Table 2).
Table 2.
Participant characteristics
| Study | Country | n | Age, years | Sex, % male | Sepsis, % of cohort | Medical inclusion criteria |
|---|---|---|---|---|---|---|
| Singer, 201510 | US | 160 | 71a | 58 | 100 | Included in sepsis registry, lactate ≥2, suspected infection, at least two SIRS criteria |
| Singer, 201411 | US | 160 | 71a | 58 | 100 | Included in sepsis registry, lactate ≥2, suspected infection, at least two SIRS criteria |
| Larsen, 201112 | US | 247 | 6a | 49 | 100 | Septic shock, sepsis (ICD9 code at discharge) |
| Mullen, 201413 | US | 59 | 61b | 56 | 91 | Critically ill medical patient |
| Maung, 201414 | Singapore | 865 | 62.5b | 59 | 58 | Mechanically ventilated, in shock, presented via ED |
| Choong, 201415 | Singapore | 1430 | 61.2b | 60 | 43 | Mechanically ventilated, admitted to ICU, via ED |
| Smith, 201016 | US | 210 | – | 59 | 100 | Suspected infection, critically ill, septic shock, admitted to ICU |
| Varpula, 200717 | Finland | 92 | 57a | 72 | 100 | Septic shock, admitted to ICU, community-acquired sepsis |
Median.
Mean. ED = emergency department. ICD = International Classification of Diseases. ICU = intensive care unit. SIRS = systemic inflammatory response syndrome.
Methods of point-of-care lactate testing included arterial,17 venous,10–12 and fingerprick samples,13 and were unclear in three studies.14–16 Precise timing of point-of-care lactate measurement within the care pathway was also not specified in any study. Average lactate levels ranged from 2.3 to 3.9 mmol/L in the three studies reporting this,11,16,17 with no significant differences between intervention and control groups. One study used lactate of ≥2 mmol/L as an inclusion criteria.11 No indicators of illness severity (for example, NEWS, APACHE) were available for between-study comparison.
Lactate result handling was expressly described in two of the papers. In one of these, lactate levels ≥2 were immediately communicated to the attending physician, and patients with a level of ≥4 were escalated to a critical care area; all patients were tested again after 2 hours.11
In the pre-hospital aeromedical setting, point-of-care lactate results were reported on hospital arrival to the attending physician.13
Study quality assessment and risk of bias
Study quality assessment and risk of bias is presented in Table 3. All included studies were found to have a moderate or serious risk of bias.
Table 3.
Quality assessment and risk of biasa
| Eligible Studies | Risk of bias domains — ROBINS-I | |||||||
|---|---|---|---|---|---|---|---|---|
| Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in the selection of the reported result | Overall risk of bias | |
| Singer, 201510 | Moderate | Moderate | Low | Low | NI | Low | Low | Moderate |
| Singer, 201411 | Moderate | Moderate | Low | Low | NI | Low | Low | Moderate |
| Larsen, 201112 | Moderate | Moderate | Low | Serious | NI | Moderate | Moderate | Moderate |
| Mullen, 201413 | Serious | Serious | Moderate | Low | NI | Moderate | Moderate | Serious |
| Maung, 201414 | Serious | Serious | Moderate | Low | NI | Moderate | Moderate | Serious |
| Choong, 201415 | Serious | Serious | Moderate | Low | NI | Moderate | Moderate | Serious |
| Smith, 201016 | Serious | Serious | Moderate | Low | NI | Moderate | Moderate | Serious |
| Varpula, 200717 | Serious | Serious | Moderate | Serious | NI | Moderate | Serious | Serious |
Low — comparable with a well-performed RCT. Moderate — sound for a non-randomised study but not comparable with a well-performed RCT. Serious — important problems in this domain. Critical — too problematic to provide any useful evidence on the effects of the intervention. NI = no information. RCT = randomised controlled trial.
Key limitations identified included: study design (lack of parallel group randomised trials); lack of definition of allocation to point-of-care or usual care lactate testing in prospective cohort studies; use of cohorts enriched for effect due to underlying risk (particularly in cohorts examining ED data only for patients subsequently admitted to the ICU); and potential for confounding of effects due to simultaneous introduction of wider sepsis care bundles.
Due to the study limitations identified, and lack of comparability across study cohorts and sampling methods, no valid meta-analysis of outcome data was possible, and thus outcomes are reported descriptively.
Effects on patient outcomes and healthcare processes
Mortality
Six studies examined the effect on in-hospital mortality (Table 4). Three studies reported a significant reduction in mortality with point-of-care lactate testing (mortality of 6% versus 19%, P = 0.02;11 odds ratio [OR] 0.6, P = 0.001;14 and OR 0.71, P = 0.006).15 Two studies reported a non-significant trend towards reduction in mortality.12,17 Only one study, in the aeromedical patient transport setting, did not demonstrate a trend towards reduced mortality with point-of-care lactate testing (55% versus 49%, P = 0.78).13 Two studies additionally reported decreased in-ICU mortality (OR 0.65, P = 0.004,14 and OR 0.64, P = 0.005).15
Table 4.
Effect on patient mortality
| Outcome | Study | Intervention group, n (%) | Usual care, n (%) | P-value |
|---|---|---|---|---|
| In-hospital mortality | Singer, 201411 | 5 (6) | 15 (19) | 0.02a |
| Larsen,b 201112 | 9 (5) | 6 (11) | 0.11 | |
| Mullen, 201413 | 11 (55) | 19 (49) | 0.78 | |
| Varpula, 200717 | 18 (34) | 14 (36) | 0.66 | |
| Maung, 201414 | OR 0.6, 95% CI = 0.46 to 0.8, P= 0.001,a with POCL testing | |||
| Choong, 201415 | OR 0.71, 95% CI = 0.55 to 0.9, P= 0.006,a with POCL testing |
Reaches statistical significance.
Larsen study figures derived from supplementary data provided by first author correspondence. OR = odds ratio. POCL = point-of-care lactate testing.
Time to treatment
Outcomes for intravenous (IV) fluid administration in five studies included time to IV fluids (minutes), receiving IV fluids in <1 hour, and total volume of IV fluids received (Table 5). Two studies in ED patients demonstrated a significant reduction in time taken for patients to receive IV fluids (median time of 71 versus 55 minutes, P = 0.03;11 48.8% versus 35.5% receiving IV fluids in less than an hour, P = 0.001).14 No significant difference in the total volume of IV fluids received was found in the two studies examining this (2000 mL versus 2500 mL, P = 0.71,11 and 3300 versus 5000 mL, P = 0.79).13
Table 5.
Effect on time to treatment and length of stay
| Outcome | Study | Intervention group | Usual care | P-value | |
|---|---|---|---|---|---|
| Length of stay | Length of hospital stay (days) | Singer, 201411 | 7 (3–13)a | 8 (4–13)a | 0.27 |
| Larsen, 201112 | 5.8a | 7.5a | <0.05c | ||
| Length of ED stay (minutes) | Singer, 201411 | 352 (246–457)a | 326 (249–436)a | 0.5 | |
| Mullen, 201413 | 396b | 216b | 0.02c | ||
| Length of ICU stay (days) | Singer, 201411 | 3 (2–6)a | 4 (2–6)a | 0.9 | |
|
| |||||
| Lactate result | Time to lactate result (minutes) | Singer, 201411 | 34 (26–55)a | 122 (82–149)a | <0.001c |
| Lactate result in <1 hour | Smith, 201016 | OR 4.6, 95% CI = 1.8 to 11.5, with POCL testing | |||
|
| |||||
| IV fluids | Time to IV fluids (minutes) | Singer, 201411 | 55 (34–83)a | 71 (42–110)a | 0.03c |
| IV fluids in <1 hour (%) | Maung, 201414 | 48.8 | 35.5 | 0.001c | |
| Total volume IV fluids (mL) | Singer, 201411 | 2000 (2000–3125)a | 2500 (2000–4000)a | 0.71 | |
| Mullen, 201413 | 3300 | 5000 | 0.79 | ||
| IV fluids (ml/kg) | Smith, 201016 | 29.3 ± 3.4b | 17.8 ± 1.4b | <0.01c | |
|
| |||||
| Antibiotics | Time to Abx (minutes) | Singer, 201411 | 89 (63–182)a | 97 (55–160)a | 0.59 |
| Abx in <1 hour (%) | Maung, 201414 | 25 | 15.1 | 0.007c | |
| Abx in <3 hours | Smith, 201016 | OR 4.2, 95% CI = 1.2 to 14.4, with POCL testing | |||
| Transfusion | Received transfusion (%) | Mullen, 201413 | 50 | 62 | 0.41 |
Median (IQR).
Mean.
Reaches statistical significance. Abx = antibiotics. CI = confidence interval. ED = emergency department. ICU = intensive care unit. IQR = interquartile range. IV = intravenous. OR = odds ratio. POCL = point-of-care lactate.
Two studies of adult patients in ED demonstrated a statistically significant reduction in time to antibiotic administration with point-of-care lactate testing, with one study demonstrating 25% of patients receiving antibiotics in <1 hour compared with 15.1% (P = 0.007),14 and a second study quoting an odds ratio of 4.2 (95% confidence interval [CI] = 1.2 to 14.4) for receiving antibiotics in <3 hours.16 However, a third study in a similar setting failed to find any significant difference in time to antibiotic administration (median time of 89 versus 97 minutes, P = 0.59).11
No significant change in the number of patients receiving blood transfusions, intubation, or central venous catheter (CVC) line insertion (nor time to CVC line insertion), were demonstrated in adult patients,13 although one study did report an odds ratio of 9.8 (95% CI = 3.5 to 27.4) for measurement of central venous pressure (CVP) in ED with the introduction of point-of-care lactate testing.16
In the study of a paediatric population, the proportion of children receiving a fluid bolus within both 1 hour and 15 minutes of ED arrival was significantly increased by implementation of an ED septic shock protocol and care guideline, which included a point-of-care lactate measurement (43% versus 79%, and 10% versus 47% respectively, P<0.05).12 An improvement in the proportion of paediatric patients receiving antibiotics in <3 hours was also evident following implementation of this sepsis protocol. However, insufficient data were available for sub-analysis of any effect due to point-of-care lactate testing alone.12
Length of stay
Three studies11–13 examined length of stay (Table 5). One demonstrated a significant reduction in median length of paediatric hospital stay, from 7.5 to 5.8 days (P<0.05).12 Another11 found no significant difference in duration across total hospital, ED, or ICU length of stay; median hospital stay was 1 day less in patients with point-of-care lactate testing (7 versus 8 days, P = 0.27), although rates of admission to the ICU were significantly lower in patients who had received point-of-care lactate testing (33% versus 51%, P = 0.02).11
A third study, in the pre-hospital aeromedical setting, demonstrated a significant increase in ED length of stay in the intervention group with pre-arrival point-of-care testing, from a mean time of 216 to 396 minutes (P = 0.02).13 However, they do not report subsequent hospital admission or length of stay following this.
Time to available lactate result
Two studies compared point-of-care to laboratory lactate testing. One study demonstrated a significant reduction in time-to-lactate result (from time of arrival) from a median of 122 minutes to 34 minutes in an ED setting (P<0.001).11 A second study quoted an OR of 4.6 (95% CI = 1.8 to 11.5) for acquiring a lactate result in <1 hour when using point-of-care testing (Table 5).16
DISCUSSION
Summary
This review identifies an important gap in the evidence needed to guide community clinicians regarding the clinical benefit of point-of-care lactate testing for suspected sepsis in community settings. There were no randomised controlled trials and no studies in primary care. The observational studies identified suffered from serious limitations, and represented very heterogeneous study populations. The majority of included patients were severely unwell, with confirmed sepsis or septic shock, and a high proportion were admitted to ICU and mechanically ventilated.
However, available evidence suggests that point-of-care lactate testing was associated with a trend towards decreased subsequent in-hospital mortality. The authors found that point-of-care lactate testing at initial assessment was associated with a reduction in the time to IV fluids and, in two studies, time to IV antibiotics, as well as an expected reduction in time to result compared with laboratory lactate. Sepsis is a time-critical condition. For every hour delay in IV antibiotic administration there is an estimated 8% increase in mortality.18 The authors found variable evidence of benefit on length of stay.
Strengths and limitations
This is the first systematic review to explore the evidence for the impact on mortality of point-of-care lactate testing in suspected sepsis at initial healthcare assessment. The authors undertook a comprehensive literature search that is unlikely to have missed relevant studies.
There are several limitations. There were no studies set in primary care (including out-of-hours general practice) and only one study in a pre-hospital (highly specialised aeromedical) setting,13 which was managing critically unwell patients. The remaining studies reported findings from data collected from emergency department patients, of which only four included patients subsequently transferred to ICU. There are likely to be substantial differences, most notably in severity of illness, between patients presenting to emergency departments and those presenting to primary care. It is unclear to what extent the results from this study can be extrapolated to these settings.
In addition, no RCTs were identified. The results presented, therefore, only suggest association between the intervention and outcome with no evidence of causality. In two of the studies,12,17 point-of-care lactate was introduced alongside a number of additional interventions aimed at reducing mortality from sepsis, and it was not possible to determine the contribution of point-of-care lactate alone to improvements observed. A single paper12 looked at a paediatric population and therefore the authors were unable to assess the influence of age on outcomes.
Comparison with existing literature
The NICE sepsis guidance from 2016 highlights that systems need to extend to primary care to facilitate early recognition and prompt treatment, and transfer of patients to the most appropriate location of care in a timely fashion.2 However, the identification of sepsis in a general practice setting can be challenging. Use of vital sign recording has been highlighted as a key way to improve sepsis recognition in the community,2,4,7,19 although the utility of scoring systems in primary care to identify sick patients is still debated.2 Despite this, there will still be some patients with sepsis with normal observations that are missed. These individuals may however have an elevated lactate (cryptic shock), and evidence suggests their mortality rate is as high as in those with overt septic shock.19–21 Therefore, the addition of point-of-care lactate may be of value, and handheld meters have been suggested to be reliable when compared to laboratory-based lactate assays.22,23
Furthermore, testing may inform decisions about administration of immediate antimicrobial treatment (for the most unwell), timing and speed of transfer to hospital, or appropriateness of alternatives to hospital admission. A recent systematic review of primary care physicians described a positive approach to the potential utility of point-of-care diagnostics in reducing diagnostic certainty and increasing more effective targeting of treatment. However, it highlighted the need for reassurance about accuracy and utility of testing — and the possibility of misleading results and resultant over-treatment.24
Implications for research and practice
At present, there is a complete lack of evidence to support the use of point-of-care lactate testing in primary care, out-of-hours primary care, or ambulance settings to improve patient outcomes. Furthermore, the appropriate threshold and prognostic values for lactate may be different at first assessment in the community, given that established thresholds have been validated in secondary care cohorts with a different spectrum of illness severity, and more established pathophysiological change later in the course of an illness. Additionally, there are potential disadvantages of using such a test earlier in the pathway of care, such as increasing the time taken for assessment, false reassurance in emerging septic shock, or a much higher false positive rate in a setting of much lower prevalence leading to inappropriate care escalation. Consideration must also be given to the potential cost of equipment, reagents, and staff training in the context of a lower potential frequency of testing in primary care.
In the limited evidence base described in this review, there are trends towards reduced mortality and reduced time to treatment that point to the potential for point-of-care lactate testing to support recognition of sepsis in the community, decreasing mortality while avoiding unnecessary and costly admissions. However, despite the potential challenges of designing such a study, randomised controlled trial evidence from community settings is now required. This might include evaluation in ambulatory community or out-of-hours primary care settings, where the prevalence of sepsis is higher, or evaluation of POC lactate as an addition to a diagnostic algorithm appropriate for community settings. Such studies would have the potential to offer the robust evidence needed for this potentially beneficial diagnostic technology.
Acknowledgments
The authors would like to acknowledge Gitte Larsen, Professor of Paediatric Critical Care at the University of Utah, for sharing her primary research data, enabling its inclusion in this review.
Appendix 1. Search strategy
| Search strategy: MEDLINE database | ||
|---|---|---|
| # Δ | Searches | Results |
| 1 | Ambulatory Care/ | 38 320 |
| 2 | exp Ambulatory Care Facilities/ | 49 281 |
| 3 | general practice/ or family practice/ | 68 949 |
| 4 | general practitioners/ or physicians, family/ or physicians, primary care/ | 22 034 |
| 5 | Primary Health Care/ | 60 672 |
| 6 | Office Visits/ | 5998 |
| 7 | exp Emergency Service, Hospital/ | 57 499 |
| 8 | Emergency Medical Services/ | 35 438 |
| 9 | (ambulatory adj3 (care or setting? or facilit* or ward? or department? or service?)).ti,ab. | 14 318 |
| 10 | ((general or family) adj2 (practi* or physician? or doctor?)).ti,ab. | 101 275 |
| 11 | (primary care or primary health care or primary healthcare).ti,ab. | 100 603 |
| 12 | (emergency adj3 (care or setting? or facilit* or ward? or department? or service?)).ti,ab. | 84 890 |
| 13 | ed.ti,ab. | 43 416 |
| 14 | (after hour? or afterhour? or “out of hour?” or ooh).ti,ab. | 3657 |
| 15 | (clinic? or visit?).ti,ab. | 374 546 |
| 16 | ((health* or medical) adj2 (center? or centre?)).ti,ab. | 95 929 |
| 17 | point-of-care systems/ or point-of-care testing/ | 9213 |
| 18 | ((“point of care” or poc or rapid or “near patient” or bedside or bed-side or “same time” or “same visit” or portable or handheld or hand-held) adj3 (test* or diagnos* or analys* or analyz* or assay? or monitor* or device?)).ti,ab. | 62 397 |
| 19 | (“point of care” or poc or poct).ti. | 3958 |
| 20 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 | 903 740 |
| 21 | Lactic Acid/an, bl [Analysis, Blood] | 8676 |
| 22 | Lactates/an, bl [Analysis, Blood] | 14 443 |
| 23 | (lactate? or lactic acid).ti,ab. | 108 786 |
| 24 | 21 or 22 or 23 | 116 951 |
| 25 | exp bacterial infections/ or exp infection/ | 1 231 179 |
| 26 | (septic* or sepsis).ti,ab. | 120 136 |
| 27 | (infection? or infectious).ti,ab. | 1 214 085 |
| 28 | bacter?emi*.ti,ab. | 26 382 |
| 29 | pneumonia.ti,ab. | 92 403 |
| 30 | cellulitis.ti,ab. | 7101 |
| 31 | meningitis.ti,ab. | 45 135 |
| 32 | pyelonephritis.ti,ab. | 11 629 |
| 33 | ((infective or reactive) adj2 arthritis).ti,ab. | 2434 |
| 34 | Systemic Inflammatory Response Syndrome/ | 4530 |
| 35 | Systemic Inflammatory Response Syndrome.ti,ab. | 3674 |
| 36 | 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 | 2 118 860 |
| 37 | 20 and 24 and 36 | 572 |
| 38 | ((“point of care” or poc or rapid or “near patient” or bedside or bed-side or “same time” or “same visit” or portable or handheld or hand-held) adj3 (lactate? or lactic acid)).ti,ab. | 330 |
| 39 | 37 or 38 | 876 |
| 40 | exp animals/ not humans.sh. | 4 260 612 |
| 41 | (mouse or mice or murine or rats or rat or rodent? or pig or pigs or piglet? or porcine or cattle or bull or bulls or cow or cows or calf or calves or bovine or sheep or ewe or ewes or ovine or horse? or equine or dog or dogs or canine or cat or cats or feline or rabbit? or ruminant?).ti. | 1 964 783 |
| 42 | 40 or 41 | 4 561 022 |
| 43 | 39 not 42 | 738 |
| Search strategy: Embase database | ||
|---|---|---|
| # Δ | Searches | Results |
| 1 | ambulatory care/ | 32 546 |
| 2 | general practice/ | 74 818 |
| 3 | general practitioner/ | 73 644 |
| 4 | Primary Health Care/ | 52 145 |
| 5 | Primary Medical Care/ | 74 311 |
| 6 | emergency ward/ | 83 516 |
| 7 | (ambulatory adj3 (care or setting? or facilit* or ward? or department? or service?)).ti,ab. | 17 971 |
| 8 | ((general or family) adj2 (practi* or physician? or doctor?)).ti,ab. | 126 166 |
| 9 | (primary care or primary health care or primary healthcare).ti,ab. | 124 505 |
| 10 | (emergency adj3 (care or setting? or facilit* or ward? or department? or service?)).ti,ab. | 116 714 |
| 11 | ed.ti,ab. | 68 889 |
| 12 | (after hour? or afterhour? or “out of hour?” or ooh).ti,ab. | 4895 |
| 13 | (clinic? or visit?).ti,ab. | 544 944 |
| 14 | ((health* or medical) adj2 (center? or centre?)).ti,ab. | 127 269 |
| 15 | “point of care testing”/ | 7014 |
| 16 | ((“point of care” or poc or rapid or “near patient” or bedside or bed-side or “same time” or “same visit” or portable or handheld or hand-held) adj3 (test* or diagnos* or analys* or analyz* or assay? or monitor* or device?)).ti,ab. | 78 227 |
| 17 | (“point of care” or poc or poct).ti. | 5335 |
| 18 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 | 1 169 598 |
| 19 | *lactic acid/ | 17 557 |
| 20 | (lactate? or lactic acid).ti,ab. | 128 852 |
| 21 | 19 or 20 | 132 107 |
| 22 | exp *infection/ | 1 957 032 |
| 23 | (septic* or sepsis).ti,ab. | 162 521 |
| 24 | (infection? or infectious).ti,ab. | 1 491 393 |
| 25 | bacter?emi*.ti,ab. | 32 883 |
| 26 | pneumonia.ti,ab. | 125 628 |
| 27 | cellulitis.ti,ab. | 9542 |
| 28 | meningitis.ti,ab. | 53 217 |
| 29 | pyelonephritis.ti,ab. | 14 281 |
| 30 | ((infective or reactive) adj2 arthritis).ti,ab. | 3058 |
| 31 | systemic inflammatory response syndrome/ | 8693 |
| 32 | Systemic Inflammatory Response Syndrome.ti,ab. | 5070 |
| 33 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 2 839 449 |
| 34 | 18 and 21 and 33 | 1275 |
| 35 | ((“point of care” or poc or rapid or “near patient” or bedside or bed-side or “same time” or “same visit” or portable or handheld or hand-held) adj3 (lactate? or lactic acid)).ti,ab. | 431 |
| 36 | 34 or 35 | 1651 |
| 37 | (exp animals/ or nonhuman/) not human/ | 5 979 837 |
| 38 | (mouse or mice or murine or rats or rat or rodent? or pig or pigs or piglet? or porcine or cattle or bull or bulls or cow or cows or calf or calves or bovine or sheep or ewe or ewes or ovine or horse? or equine or dog or dogs or canine or cat or cats or feline or rabbit? or ruminant?).ti. | 2 200 031 |
| 39 | 37 or 38 | 6 276 204 |
| 40 | 36 not 39 | 1472 |
| Search strategy: Cochrane database | |
|---|---|
| #1 | MeSH descriptor: [Lactates] explode all trees |
| #2 | lactate* or “lactic acid”:ti,ab,kw (Word variations have been searched) |
| #3 | #1 or #2 |
| #4 | MeSH descriptor: [Ambulatory Care] this term only |
| #5 | MeSH descriptor: [Ambulatory Care Facilities] explode all trees |
| #6 | MeSH descriptor: [General Practice] explode all trees |
| #7 | MeSH descriptor: [General Practitioners] explode all trees |
| #8 | MeSH descriptor: [Physicians, Family] explode all trees |
| #9 | MeSH descriptor: [Physicians, Primary Care] explode all trees |
| #10 | MeSH descriptor: [Primary Health Care] this term only |
| #11 | MeSH descriptor: [Office Visits] this term only |
| #12 | MeSH descriptor: [Emergency Service, Hospital] explode all trees |
| #13 | MeSH descriptor: [Emergency Medical Services] this term only |
| #14 | (ambulatory near/3 (care or setting* or facilit* or ward* or department* or service*)):ti,ab,kw (Word variations have been searched) |
| #15 | ((general or family) near/2 (practi* or physician* or doctor*)):ti,ab,kw (Word variations have been searched) |
| #16 | primary care or “primary health care” or “primary healthcare”:ti,ab,kw (Word variations have been searched) |
| #17 | (emergency near/3 (care or setting* or facilit* or ward* or department* or service*)):ti,ab,kw (Word variations have been searched) |
| #18 | ed:ti,ab,kw (Word variations have been searched) |
| #19 | after hour* or afterhour* or “out of hour*” or ooh:ti,ab,kw (Word variations have been searched) |
| #20 | clinic or clinics or visit or visits:ti,ab,kw (Word variations have been searched) |
| #21 | ((health* or medical) adj2 (center* or centre*)):ti,ab,kw (Word variations have been searched) |
| #22 | MeSH descriptor: [Point-of-Care Systems] explode all trees |
| #23 | ((“point of care” or poc or rapid or “near patient” or bedside or bed-side or “same time” or “same visit” or portable or handheld or hand-held) near/3 (test* or diagnos* or analys* or analyz* or assay* or monitor* or device*)):ti,ab,kw (Word variations have been searched) |
| #24 | point of care or poc or poct:ti (Word variations have been searched) |
| #25 | #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 |
| #26 | #3 and #25 |
| #27 | ((“point of care” or poc or rapid or “near patient” or bedside or bed-side or “same time” or “same visit” or portable or handheld or hand-held) near/3 (lactate* or “lactic acid”)):ti,ab,kw (Word variations have been searched) |
| #28 | #26 or #27 |
| Search strategy: Web of Science database | ||
|---|---|---|
| Set | ||
| Results | Save search history and/or create an alertOpen a saved search history | |
| # 8 | 1134 | #7 OR #6 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 7 | 521 | TOPIC: (((“point of care” or poc or rapid or “near patient” or bedside or bed-side or “same time” or “same visit” or portable or handheld or hand-held) NEAR/3 (lactate* or “lactic acid”))) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 6 | 666 | #5 AND #4 AND #3 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 5 | 1 395 723 | TOPIC: (infection* OR infectious) OR TOPIC: (bacteremia OR bacteraemia OR septic OR sepsis) OR TOPIC: (pneumonia OR cellulitis OR meningitis OR pyelonephritis) OR TOPIC: ((infective or reactive) NEAR/2 arthritis) OR TOPIC: (“Systemic Inflammatory Response Syndrome”) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 4 | 134 718 | TOPIC: (lactate* or “lactic acid”) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 3 | 664 586 | #2 OR #1 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 2 | 93 998 | TOPIC: (((“point of care” or poc or rapid or “near patient” or bedside or bed-side or “same time” or “same visit” or portable or handheld or hand-held) near/3 (test* or diagnos* or analys* or analyz* or assay* or monitor* or device*))) |
| # 1 | 575 763 | medical) NEAR/2 (center* or centre*))) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
Funding
This work presents independent research funded by the National Institute for Health Research (NIHR) Diagnostic Evidence Cooperative Oxford. David McCartney is supported by an NIHR in-practice fellowship (IPF). Elizabeth Morris is supported by a Nuffield Department of Primary Care Health Sciences academic clinical fellowship (ACF). Rebecca Fisher was supported by an NIHR ACF at the time of this work. Daniel Lasserson is supported by the NIHR Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Ethical approval
Not applicable.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence. Sepsis: recognition, diagnosis and early management. NG51. London: NICE; 2016. https://www.nice.org.uk/guidance/NG51 (accessed 9 Oct 2017) [PubMed] [Google Scholar]
- 3.Daniels R, Nutbeam T, McNamara G, Galvin C. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J. 2011;28(6):507–512. doi: 10.1136/emj.2010.095067. [DOI] [PubMed] [Google Scholar]
- 4.Gilham C. Sepsis: the primary care focus. Br J Gen Pract. 2016 doi: 10.3399/bjgp16X683905. https://doi.org/10.3399/bjgp16X683905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Society of Critical Care Medicine Surviving sepsis campaign. http://www.survivingsepsis.org/Pages/default.aspx (accessed 9 Oct 2017)
- 6.Royal College of General Practitioners Clinical priority programme: sepsis: April 2017 to March 2019. http://www.rcgp.org.uk/clinical-and-research/our-programmes/clinical-priorities.aspx (accessed 9 Oct 2017)
- 7.NHS England Improving outcomes for patients with sepsis A cross-system action plan. 2015. https://www.england.nhs.uk/wp-content/uploads/2015/08/Sepsis-Action-Plan-23.12.15-v1.pdf (accessed 9 Oct 2017)
- 8.Howick J, Cals JWL, Jones C, et al. Current and future use of point-of-care tests in primary care: an international survey in Australia, Belgium, the Netherlands, the UK and the USA. BMJ Open. 2014;4:e005611. doi: 10.1136/bmjopen-2014-005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer AJ, Chase K, Murphy P, Thode HC., Jr The financial effect of point-of-care lactates in ED patients with sepsis. [Conference abstract] Acad Emerg Med. 2015;1:S385. http://onlinelibrary.wiley.com/doi/10.1111/acem.12644/epdf (accessed 17 Oct 2017). [Google Scholar]
- 11.Singer AJ, Taylor M, LeBlanc D, et al. ED bedside point-of-care lactate in patients with suspected sepsis is associated with reduced time to IV fluids and mortality. Am J Emerg Med. 2014;32(9):1120–1124. doi: 10.1016/j.ajem.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Larsen GY, Mecham N, Greenberg R. An emergency department septic shock protocol and care guideline for children initiated at triage. Pediatrics. 2011;127(6):e1585–e1592. doi: 10.1542/peds.2010-3513. [DOI] [PubMed] [Google Scholar]
- 13.Mullen M, Cerri G, Murray R, et al. Use of point-of-care lactate in the prehospital aeromedical environment. Prehosp Disaster Med. 2014;29(2):200–203. doi: 10.1017/S1049023X13009254. [DOI] [PubMed] [Google Scholar]
- 14.Maung LH, Ng XH, Ong ZY, See KC. Early lactate measurement triggers early treatment and improves outcomes in critically ill patients. [Conference abstract] Ann Acad Med Singapore. 2014;1:S327. http://www.annals.edu.sg/pdf/43VolNo9Sep2014/SHBC2014_Final.pdf (accessed 17 Oct 2017). [Google Scholar]
- 15.Choong See K, Dizon J, Mahadevan M, et al. Impact of point-of-care lactate measurement on mortality in critically-ill patients requiring mechanical ventilation. European Respiratory Society Annual Congress. Eur Respir J. 2014;44:P2062. [Google Scholar]
- 16.Smith S, Russi CS, Kashyap R, et al. Point-of-care lactate measurement in an emergency department is associated with expedited early goal-directed management of severe sepsis and septic shock. [Conference abstract] Am J Respir Crit Care Med. 2010;181:A6141. http://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A6141 (accessed 17 Oct 2017). [Google Scholar]
- 17.Varpula M, Karlsson S, Parviainen I, et al. Community-acquired septic shock: early management and outcome in a nationwide study in Finland. Acta Anaesthesiol Scand. 2007;51(10):1320–1326. doi: 10.1111/j.1399-6576.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 19.UK Sepsis Trust Toolkit: general practice recognition & management of sepsis in adults and children and young people over 12 years — 2016. https://sepsistrust.org/wp-content/uploads/2017/08/GP-toolkit-2016-FINAL-2.pdf (accessed 17 Oct 2017)
- 20.Bakker J, Jansen TC. Don’t take vitals, take a lactate. Intensive Care Med. 2007;33(11):1863–1865. doi: 10.1007/s00134-007-0679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro NI, Fisher C, Donnino M, et al. The feasibility and accuracy of point-of-care lactate measurement in emergency department patients with suspected infection. J Emerg Med. 2010;39(1):89–94. doi: 10.1016/j.jemermed.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismail F, Mackay WG, Kerry A, et al. The accuracy and timeliness of a point of care lactate measurement in patients with sepsis. Scand J Trauma Resusc Emerg Med. 2015;23:68. doi: 10.1186/s13049-015-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones CH, Howick J, Roberts NW, et al. Primary care clinicians’ attitudes towards point-of-care blood testing: a systematic review of qualitative studies. BMC Fam Pract. 2013;14(1):117. doi: 10.1186/1471-2296-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]