Figure 1.
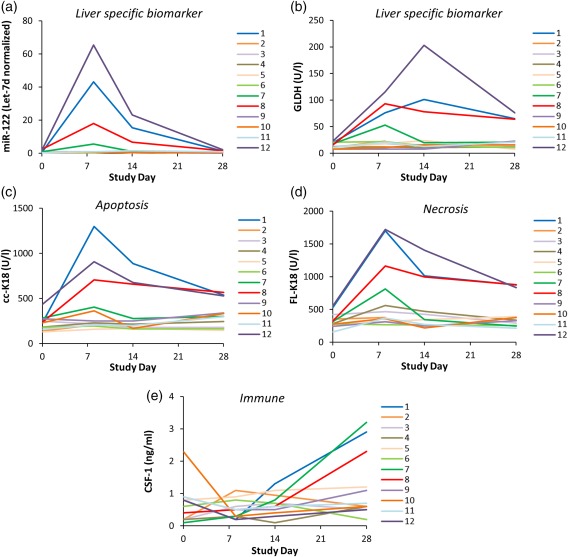
Serum levels of liver safety biomarkers (a) microRNA‐122 (miR‐122), (b) glutamate dehydrogenase (GLDH), (c) caspase‐cleaved fragment of K18 (cc‐K18), (d) full length keratin‐18 (FL‐K18), and (e) macrophage colony stimulating factor (CSF‐1). Serum samples were collected at four different timepoints relative to cimaglermin alfa (or placebo) administration from 12 patients who participated in phase I clinical trials including two patients which met FDA drug‐induced liver injury (DILI) guidance stopping criteria (Subjects 8 and 12), as well as others with elevated serum aminotransferases that fell short of meeting FDA DILI guidance stopping criteria (Subjects 1, 2, 3, 4, 7, 9, 10, 11), and two placebo‐dosed individuals (Subjects 5 and 6).
