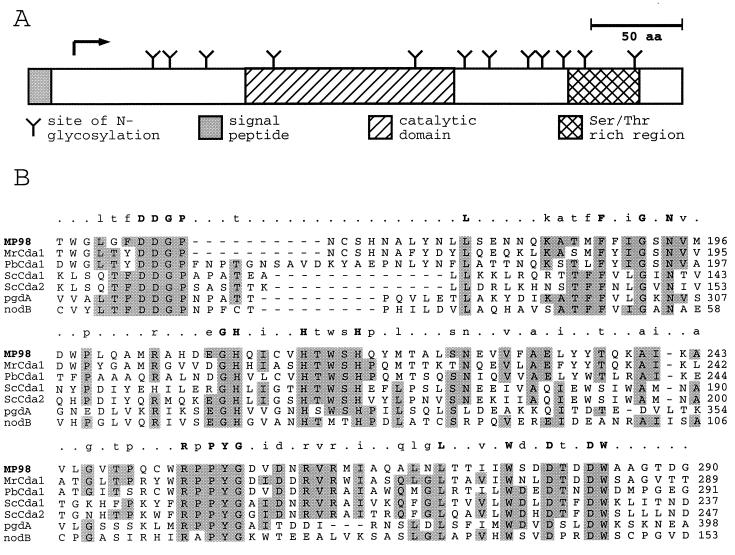
Figure 4.
Features of the MP98 protein sequence (A) and its similarity to N-acetylglucosamine deacetylases (B). The signal sequence (shaded), catalytic domain (hatched), and Ser/Thr rich region (cross-hatch) of MP98 are boxed in A. Each putative site of N-glycosylation is indicated by a Y, and the position of the N terminus of the mature MP98 protein is indicated by an arrow. The putative catalytic domain of MP98 identified by blast searches was aligned by clustal with comparable regions of chitin deacetylases of M. rouxii (MrCDA1, A47713); P. blakesleeanus (PbCDA1, BAB03595); S. cerevisiae (ScCDA1, NP 013410 and ScCDA2, NP 013411); a peptidoglycan N-acetylglucosamine deacetylase of S. pneumonia (pgdA, CAB96552); and a chitooligosaccharide deacetylase of R. leguminosarum (nodB, P04676). Amino acids identical in four or more sequences are shaded. Above each section of the alignment amino acid identities in 6/6 sequences (uppercase letters), 4/6 or 5/6 sequences (lowercase letters), and nonidentity (periods) are summarized.