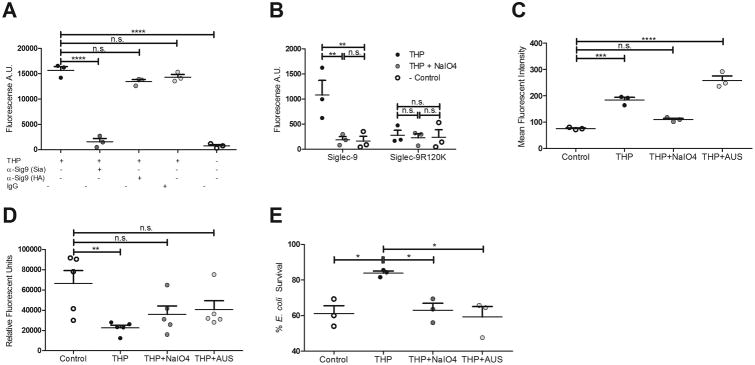
Figure 2. THP engagement of Siglec-9 and neutrophil suppression requires sialic acid.
(A) Plate-based binding assay of immobilized human Siglec-9-Fc, blocked with anti-Siglec-9 (sialic acid blocking, Sia), anti-Siglec-9 (Hyaluronic acid blocking, HA), or human IgG, and incubated with soluble THP. (B) Plate-based binding assay of immobilized mock-treated THP, NaIO4-treated THP, or no THP (Control), with human Siglec-9-Fc or Siglec-9 R120K-Fc mutant and visualized with PE anti-human IgG Fc antibody. (C) Mean fluorescent intensity of neutrophils treated with THP, sialidase-treated THP, or no THP (Control), stained with FITC mouse anti-human THP antibody. (D) ROS production of neutrophils treated with THP, NaIO4-treated THP, sialidase-treated THP, or no THP (Control), and stimulated with PMA. (E) Percent survival (left) or total CFU (right) of E. coli UTI89 after 30 min of exposure to neutrophils pretreated with THP, NaIO4-treated THP, sialidase-treated THP, or no THP (Control). Data represent the mean and SEM of three (or five in panel D) independent experiments performed in technical triplicate with combined results shown, n = 3/group (A-C, E) or n = 5/group (D). Data was analyzed using one-way ANOVA with Bonferroni's multiple comparisons post-test (A, C-E) or two-way ANOVA with Bonferroni's multiple comparisons post-test (B). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 represent statistical significance, or n.s. represents non-significant (P > 0.05).