Abstract
Memory retrieval requires coordinated intra- and inter-regional activity in networks of brain structures. Dysfunction of these networks and memory impairment are seen in many psychiatric disorders, but relatively little is known about how memory retrieval and memory failure are represented at the level of local and regional oscillatory activity. To address this question, we measured local field potentials (LFPs) from mice as they explored a novel context, retrieved memories for contextual fear conditioning, and after administration of two amnestic agents: the NMDA receptor antagonist MK-801 and muscarinic acetylcholine receptor antagonist scopolamine (SCOP). LFPs were simultaneously recorded from retrosplenial cortex (RSC), dorsal hippocampus (DH), and anterior cingulate cortex (ACC), which are involved in processing contextual memories, and analyzed for changes in intra-regional power and inter-regional peak coherence of oscillations across multiple frequency bands. Context encoding and memory retrieval sessions yielded similar patterns of changes across all three structures, including decreased delta power and increased theta peak coherence. Baseline effects of MK-801 and SCOP were primarily targeted to gamma oscillations, but in opposite directions. Both drugs also blocked memory retrieval, as indicated by reduced freezing when mice were returned to the conditioning context, but this common behavioral impairment was only associated with power and peak coherence disruptions after MK-801 treatment. These findings point to neural signatures for memory impairment, whose underlying mechanisms may serve as therapeutic targets for related psychiatric disorders.
Introduction
Memory retrieval requires the coordination of intra- and inter-regional activity in networks of brain structures. The default mode network (DMN; Raichle et al., 2001) is one such network, comprising a number of brain regions, including RSC, DH, and ACC, which are functionally and anatomically connected. Co-activation of these regions is observed during a number of cognitive tasks, such as the retrieval of episodic memories (Andrews-Hanna, 2012; Buckner et al., 2008; Spreng et al., 2009), and amnestic drugs that block memory retrieval alter the activity of DMN-associated brain regions (Honey et al., 2005; Sannita et al., 1987). Dysfunction in the DMN has been associated with amnesia, cognitive decline, and pathological states (Broyd et al., 2009; Grimm et al., 2009, Hamilton et al., 2011, Tao et al., 2015, Yu et al., 2013), highlighting the continuing need to better understand how network activity in the brain is generated and how it relates to memory retrieval and other cognitive functions.
Functional connectivity studies in rodents have identified a DMN-like network of structures (Gozzi et al., 2016; Lu et al., 2012; Sierakowiak et al., 2015) that also includes RSC, DH, and ACC. Activity within these individual structures (Anagnostaras et al., 1999; Corcoran et al., 2011, 2013; Frankland et al., 2004) and coherence of neural oscillations between structures (Corcoran et al., 2016) are associated with the retrieval of context-dependent, episodic-like memories. Retrieval of such memories can be blocked by drugs such as MK-801 (Harrod et al., 2001) and scopolamine (SCOP; Watts et al., 1981), even though these drugs act on completely distinct neurotransmitter systems. Despite these similar effects on memory retrieval (and other cognitive/emotional processes; Autry et al., 2011; Navarria et al., 2015), it is not known whether they exert similar effects on network properties within and between memory-related brain regions.
Episodic memories are particularly dependent upon hippocampal-cortical interactions (Kim, 2016). This network activity may help define brain states, such as consciousness, arousal, and emotional state, which are permissive for successful memory retrieval. These psychological processes have all been associated with patterns of oscillatory activity embedded in local field potentials (LFPs). Because the processes associated with these oscillations are interrelated, and our regions of interest (RSC, DH, and ACC) are interconnected, we recorded LFPs from all three regions, and examined oscillatory activity across six frequency bands: delta (1–4Hz), low (4–8Hz) and high (8–12Hz) theta, beta (13–30Hz), and low (30–55Hz) and high (55–80Hz) gamma. Intra-regional power and inter-regional coherence were converted to state-space vectors, allowing us to identify specific patterns of oscillations at which such network-level coordination occurs in three experiments: 1) during encoding of context memory, 2) during retrieval of memory for contextual fear conditioning, and 3) during retrieval testing after injection of MK-801 and SCOP, drugs that block memory retrieval.
Methods
Subjects
A total of forty-four nine-week-old male C57BL6/N mice obtained from a commercial supplier (Harlan, Indianapolis, IN) were used in this study. Mice were individually housed in a facility on a 12/12hr light/dark cycle (lights on at 7a.m.), and allowed free access to food and water. All procedures were approved by Northwestern University’s Animal Care and Use Committee in compliance with National Institutes of Health standards.
Surgery
Mice were anesthetized with Avertin (1.2%) and implanted with insulated silver wires (100μm diameter) aimed at RSC (1.8mm posterior, 0.4mm lateral, 0.75mm ventral to bregma), DH (1.5mm posterior, 1.0mm lateral, 1.75mm ventral), and ACC (1.3mm anterior, 0.4mm lateral, 1.75mm ventral). All electrodes were placed in the left hemisphere. A gold screw lowered into the skull near the right parietal/occipital bone suture served as a reference and ground electrode. Two stainless steel jeweler’s screws were inserted in the skull to anchor the headcap. All wires were soldered to a 6-pin connector to which the recording devices were later attached, and the assembly was fixed to the skull with acrylic. Mice were allowed at least 72h to recover from surgery prior to behavioral procedures. At the end of behavioral testing, electrode placements were verified using Nissl-stained coronal sections taken from RSC, ACC, and DH.
Context enocoding, fear conditioning, and memory retrieval testing
All behavioral testing occurred in a 35×20×20cm Plexiglas conditioning chamber with a stainless steel rod floor (4mm diameter, 0.9cm center-to-center) in a sound-attenuating cabinet with black inner walls (TSE Systems Inc., Bad Homburg, Germany). For context encoding, naïve mice were placed in the novel chamber for 3min and returned to their home cages. Contextual fear conditioning occurred the following day, and consisted of mice being placed back in the chamber for 3min, followed by presentation of a mild footshock (2s, 0.7mA, constant current). Testing for memory retrieval in the conditioning context consisted of a 3min session during which no shocks were presented. For drug testing, mice were not exposed to the conditioning chamber prior to fear conditioning. On every day, the chamber was cleaned after each mouse with 70% ethanol.
LFP acquisition
On each test day, LFP recordings began as soon as the mice were connected to wireless 4-channel NeuroLogger recording devices (TSE Systems), and continued until the end of each test session (up to 55min total). Continuous recordings were made with a sampling rate of 500Hz. Pre-amplification, analog-to-digital conversion (unity gain buffer, AC input range ±750μV, 1000x gain, ADC resolution 8bits), and data storage all occurred on the NeuroLogger. After each session, the NeuroLogger was removed and data were downloaded to a computer for later analysis.
Drugs
Mice were injected (0.2mL i.p.) with saline (0.9%), MK-801 (0.10mg/kg; Sigma, St. Louis, MO), and scopolamine (SCOP; 2.0mg/kg; Sigma). MK-801 and SCOP were dissolved in 0.9% saline. Injections were made ≈34 minutes prior to memory retrieval tests in the conditioning context. Each mouse received each injection on separate days. The order of injections was the same for all mice; injections were separated by 1–7d to allow for washout prior to the subsequent test.
Data collection and analysis
LFP recordings were converted to a Matlab-compatible format for spectral analyses using open-source Chronux algorithms (http://Chronux.org; see Rojas-Líbano et al., 2014 for a detailed description). Power and coherence spectra were computed for the delta (1–4Hz), low theta (4–8Hz), high theta (8–12Hz), beta (13–30Hz), low gamma (30–55Hz), and high gamma (55–80Hz) frequency bands across each 3min recording session using 35 half-overlapping 10s windows with 4 tapers (resulting in a frequency resolution of 1.4Hz). Coherence was transformed to z-coherence using the inverse hyperbolic tangent transform as described by Kay and Freeman (1998). There was no filtering. The frequency within each band at which coherence was highest was taken as the center frequency, and coherence at this peak was used as the dependent measure.
Although our LFP recording sessions lasted up to 55 minutes, we focused our analyses on 3min subsets of the total recordings. For context encoding and retrieval test days (Fig. 1), we focused our analyses on the 3min period before mice were exposed to the context and during the 3min context exposure. On each drug test day (Fig. 3–4), we focused our analyses on the 3min period before drug injection, a 3min period beginning 30min post-injection, and during the 3min test in the conditioning chamber. No recordings were made on the fear conditioning day.
Average power and peak coherence within each frequency band were calculated for each mouse in each session, and then converted to ratios to determine between-session changes using the formula XS2/(XS1 + XS2), where X is power or peak coherence within each band, and S1 and S2 are the recording sessions being compared (e.g., pre- and post-injection in the home cage). Thus, a ratio of 0.5 indicates no difference between recording sessions. These ratios were analyzed using two-way ANOVA, with factors of frequency band and region (for power) or site-pair (for peak coherence). Significant interaction effects indicated differences in the patterns of power and peak coherence ratios between regions/site-pairs across frequency bands, and were followed by post hoc one-sample t tests to compare power and peak coherence ratios for each region/site-pair against 0.5 to determine significant changes between recording sessions. Where interaction effects were non-significant, we only highlight instances where there was both a significant main effect of frequency band and all three regions/site-pairs showed a consistent and significant difference from 0.5 within at least one frequency band.
To better quantify differences in LFP activity between experimental conditions, we z-scored each of the 36 LFP variables (6 frequency bands x 3 brain regions x 2 measurements [power and peak coherence]) across each subject and then created LFP state vectors for each recording session containing all 36 LFP variables. One mouse was removed from the analysis as an outlier. To quantify the similarity of LFP states observed during each experimental condition, we computed the standardized Euclidean distance between the 36-dimensional clusters of state vectors belonging to each experimental condition (e.g., the distance between encoding sessions and retrieval sessions). Distances between clusters were calculated by averaging, over all vectors, the distance from each vector to the mean of the opposite cluster minus the distance to the like cluster divided by the sum of both distances. This gives the proportion of variability among state vectors that is due to differences between conditions, with higher values corresponding to lower similarity between the LFP states observed in each experimental condition. These values were then compared against zero (i.e. LFP state vectors are equidistant from the two condition means) using one-sample t tests. Significant positive values indicated that two clusters were dissimilar, whereas values not different from zero and negative values indicate similarity between clusters.
Freezing during tests for fear to the conditioning context was scored every 5s by a trained observer, and expressed as the percentage of the total number of observations that the mice remained motionless. Locomotor activity in the chamber was recorded automatically as infrared beam crosses. Between-day, within-subjects differences in post-drug freezing behavior and locomotion were analyzed using one-way ANOVA, followed by Tukey HSD post hoc tests.
Statistical differences were considered significant if the p values obtained were less than 0.05 for ANOVAs and unpaired t tests, and less than 0.01 for one-sample t tests.
Results
Power and peak coherence changes during context encoding and memory retrieval
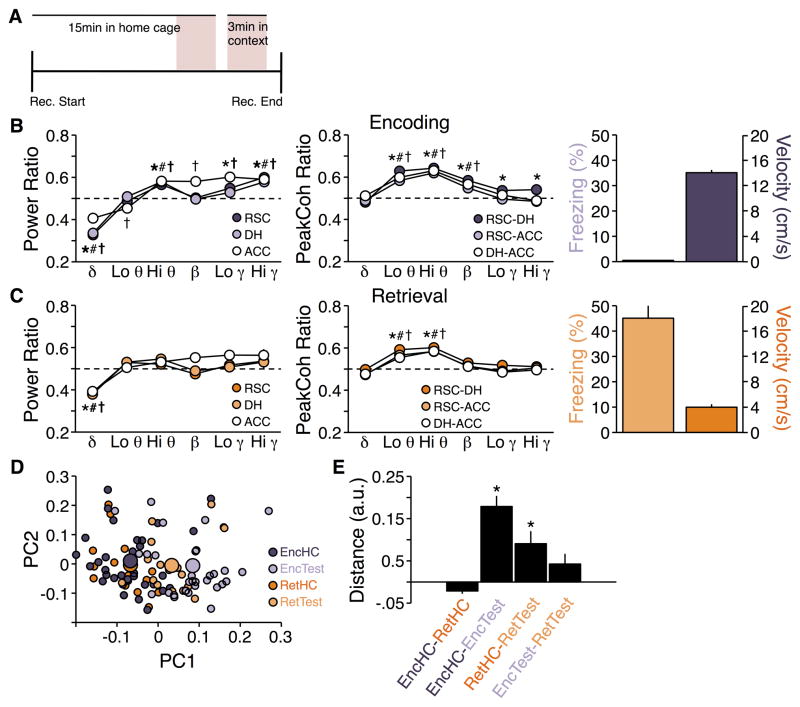
To determine power and peak coherence patterns during encoding of a context memory, we performed a meta-analysis of LFPs recorded from 33 mice exposed to a novel context as part of 6 different experiments. Power ratios across frequencies were qualitatively similar for all regions; context encoding was associated with decreased delta, increased high theta, and increased gamma power relative to the home cage (F5,480 = 145.27; p < 0.0001; Figure 1B, left). A significant interaction effect of region by frequency band (F10,480 = 5.82; p < 0.0001) suggested that there were differences across regions; post hoc tests indicated that ACC in particular showed additional changes to low theta, beta, and low gamma power. Similar to power, peak coherence ratios across frequencies were qualitatively similar for all site-pairs, with context encoding being associated with increased peak coherence in low theta, high theta, and beta peak coherence relative to the home cage (F5,480 = 137.79; p < 0.0001). Again, a significant interaction effect of site-pair by frequency band (F10,480 = 3.04; p < 0.001; Figure 1C, left) suggested that there were differences across site-pairs; post hoc tests indicated that RSC-DH peak coherence was also increased in low and high gamma.
Figure 1.
Power and peak coherence ratios during context encoding and memory retrieval. A) Timeline of recordings. LFP analyses focused on two 3 min periods (shaded pink): just prior to exposure to the conditioning chamber, and during exposure to the chamber. B) (Left) Power and (Center) peak coherence relative to home cage during context encoding. *, #, † p ≤ 0.01 vs. home cage for RSC, DH, and ACC power, respectively. Dashed lines indicate no change from home cage (ratio of 0.5). (Right) Freezing and locomotor activity were low and high, respectively, during this test. C) (Left and Center) Same as B for memory retrieval. *, #, † p ≤ 0.01 vs. home cage for RSC-DH, RSC-ACC, and DH-ACC peak coherence, respectively. (Right) Freezing and locomotor activity were high and low, respectively, during this test. D) Plot of the first two principal components derived from recordings made in the home cage (HC) and conditioning chamber (Test) on context encoding (Enc) and retrieval (Ret) days. Small symbols represent individuals in each session; large symbols represent the average for each session. E) Mean Euclidian distances between clusters in D. * Difference to hypothetical mean of 0 is non-negative and p < 0.01.
To determine power and peak coherence patterns during memory retrieval in the conditioning context, we performed a meta-analysis of LFPs recorded from 15 mice that were returned to a conditioning context as part of 5 different experiments. Qualitatively, the patterns for power (Figure 1B, right) and peak coherence (Figure 1C, right) ratios were similar to those seen during context encoding, though generally the differences from home cage appeared smaller. As was seen with encoding, ANOVA revealed main effects of frequency on power: F5,210 = 32.31 and peak coherence F5,210 = 34.07 (ps < 0.0001), but no interaction effects for either power (region by frequency; F10,210 = 1.44; p = 0.16) or peak coherence (site-pair by frequency; F10,210 = 1.91; p = 0.99). Nonetheless, all three regions showed decreased delta power as well as increased low theta and high theta peak coherence during the retrieval test.
We next ensured that the apparent similarity of activity patterns during encoding and retrieval was not an artifact of the different sample sizes in the two experiments (33 mice for encoding versus 18 mice for retrieval). We repeated the analysis of the encoding data 5 separate times, using different randomly selected subsets of 15 mice for each analysis. The patterns of power and peak coherence ratios observed in these subsets of mice (data not shown) were similar to those seen in the overall encoding meta-analysis (Figure 1B) and larger than those observed in the retrieval meta-analysis (Figure 1C), suggesting that the results of the two experiments likely did not reflect differences in sample sizes.
These analyses suggest that the patterns of LFP activity (both in terms of intra-region power and inter-region coherence) observed during the two test sessions were distinct from those observed during the home cage sessions, but similar to one another. To test this in a way that incorporated all of the available LFP information, we constructed LFP state vectors from the 36 LFP variables recorded during each session (6 frequency bands x [3 regions for power + 3 site-pairs for peak coherence]), and computed the distance between vectors from each of the experimental conditions. This analysis confirmed the trends described above. Home cage recording sessions were not different between the two test days (t47 = −3.23; p < 0.01; zero and negative t values indicate no significant difference). Encoding and retrieval test sessions were different from their respective home cage sessions (t63 = 6.22; p < 0.0001 and t31 = 3.10; p < 0.01, respectively), but similar to one another (t47 = 1.77; p = 0.083), although the distance between home cage and test session was smaller for memory retrieval than encoding (Figure 1D–E).
Although the patterns of LFP changes were similar across these two sessions, they were associated with robust behavioral differences. During encoding of context memory there was no freezing and relatively high locomotor activity (Figure 1B), whereas during retrieval of context conditioning memory, freezing was significantly increased and locomotor activity concurrently decreased (Figure 1C).
Amnestic effects of MK-801 and SCOP
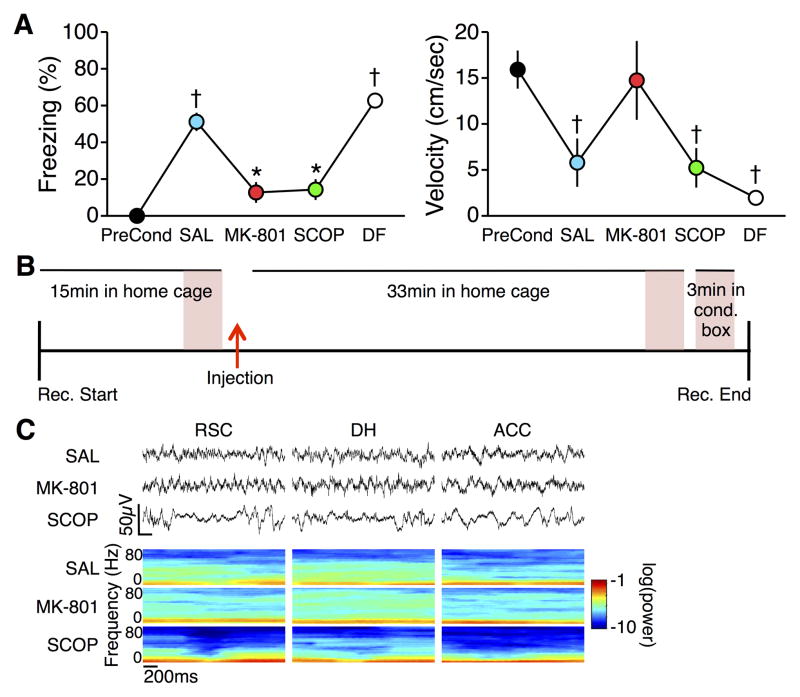
Seven mice were fear conditioned and then tested for fear to the conditioning context on four subsequent test days, with one to seven days separating each test. On each of the first three test days, mice were injected 30min prior to testing with SAL, MK-801, and SCOP. On the fourth test day, mice did not receive any injection. Both MK-801 and SCOP blocked memory retrieval, as indicated by decreased levels of freezing (F6,24 = 44.31; p < 0.0001; post hoc ps < 0.001 compared to SAL test; Figure 2A). Freezing during the subsequent drug-free test was no different than freezing during the SAL test (p = 0.30), but was significantly greater than during the MK-801 and SCOP tests (ps < 0.001), indicating that decreased freezing observed during the multiple post-drug tests was not due to extinction of the freezing response, loss of the fear conditioning memory, or an effect of the order of drug administrations. Locomotor activity was different across tests (F6,24 = 7.38; p < 0.001), but was affected differently by the two drugs. Mean velocity during the MK-801 test was not different from that during pre-conditioning (p = 1.0), and only marginally higher than during the SAL (p = 0.073) and SCOP (p = 0.051) tests. In contrast, even though freezing was low, mean velocity during the SCOP test was less than during pre-conditioning (p < 0.05) and not different from the SAL test (p = 0.76).
Figure 2.
A) (Left) Freezing during the post-injection retrieval tests. Memory retrieval was blocked by both MK-801 and SCOP, but returned to normal during a drug free (DF) test. (Right) Locomotor activity was differently affected by MK-801 and SCOP. * p < 0.001 vs. SAL; † p < 0.05 vs. preconditioning. B) Timeline of recordings. LFP analyses focused on three 3 min periods (shaded pink): just prior to drug injection, 30–33 min post injection, and during exposure to the conditioning chamber. C) Raw LFPs (Top) and LFP spectra (Bottom) from RSC, DH, and ACC during the post-injection home cage recording session.
From the LFPs collected during each of the post-injection test days, we analyzed data from three separate sessions: in the home cage prior to injection, in the home cage 30min post-injection, and in the conditioning chamber during post-injection memory retrieval testing (Figure 2B). Each injection yielded a distinct pattern of changes in LFPs recorded from all three regions (Figure 2C; no recordings were made during the drug-free test).
Drug effects on baseline power and peak coherence
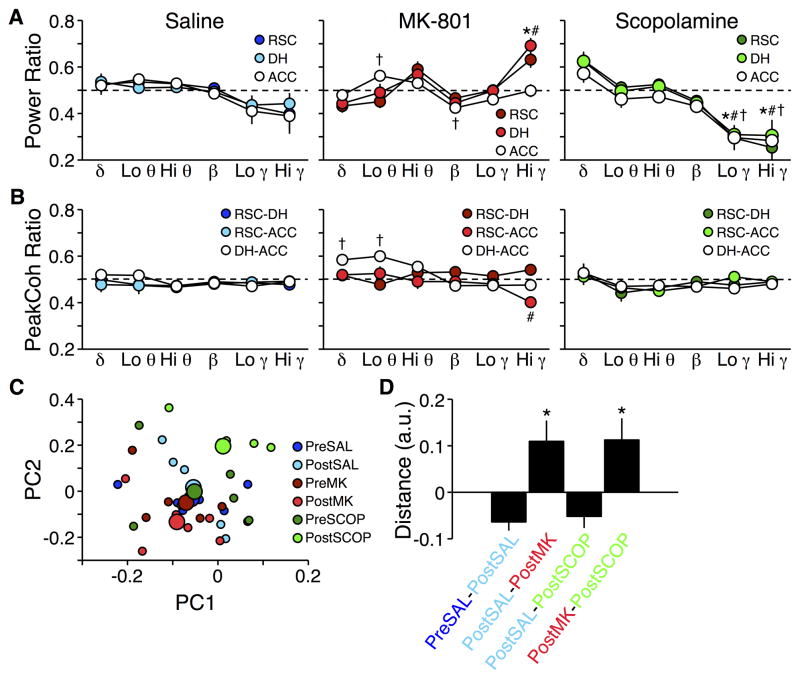
Injection of SAL had no effect on power recorded in the home cage; despite a main effect of frequency (F5,90 = 6.64; p < 0.0001), no individual region was significantly different pre- to post-injection in any frequency band (Figure 3A, left). Peak coherence was similarly unaffected (F5,90 = 1.21; p = 0.31; Figure 3B, left), and there were no interaction effects for either measurement (Fs10,90 ≤ 0.86; ps > 0.57).
Figure 3.
Power and peak coherence ratios after drug injections. A) Power during post-injection recording sessions in the home cage relative to pre-injection recording sessions in the home cage. *, #, † p ≤ 0.01 vs. home cage for RSC, DH, and ACC power, respectively. B) Same as A for peak coherence. *, #, † p ≤ 0.01 vs. home cage for RSC-DH, RSC-ACC, and DH-ACC peak coherence, respectively. Dashed lines indicate no change from pre-injection recording session (ratio of 0.5). C) Plot of the first two principal components derived from recordings made in the home cage pre- and post-injection. Small symbols represent individuals in each session; large symbols represent the average for each session. D) Mean Euclidian distances between clusters in C. * Difference to hypothetical mean of 0 is non-negative and p < 0.01.
In contrast, MK-801 produced region by frequency and site-pair by frequency interactions in power (F10,90 = 4.59; p < 0.0001; Figure 3A, center) and peak coherence (F10,90 = 6.12; p < 0.0001; Figure 3B, center), respectively. Post hoc tests revealed that, compared to pre-injection levels, low theta power increased and beta power decreased in ACC, and high gamma power increased in both RSC and DH. Peak coherence decreased in the high gamma band for the RSC-ACC site-pair, and increased in delta and low theta bands for the DH-ACC site-pair.
Similar to SAL, SCOP produced no interaction effects for either peak coherence or power (Fs10,90 < 0.60; ps > 0.81; Figure 3A–B, right), and despite a main effect of frequency band on peak coherence (F5,90 = 3.69; p < 0.01), no frequency band showed consistent changes across regions from pre-injection levels of peak coherence. However, SCOP injection did cause a decrease in both low gamma and high gamma power across all three brain regions (F5,90 = 41.20; p < 0.0001).
State-space distance analysis again confirmed the above trends. Post-SAL recordings were similar to pre-SAL (t13 = −3.54; p < 0.01), as well as to post-SCOP (t13 = −2.14; p = 0.052). MK-801, in contrast, yielded patterns of activity that were different from post-SAL (t13 = 3.42; p < 0.01). The post-MK-801 and post-SCOP tests were also different from each other (t13 = 3.10; p = 0.010; Figure 3C–D).
Drug effects on retrieval-related power and peak coherence
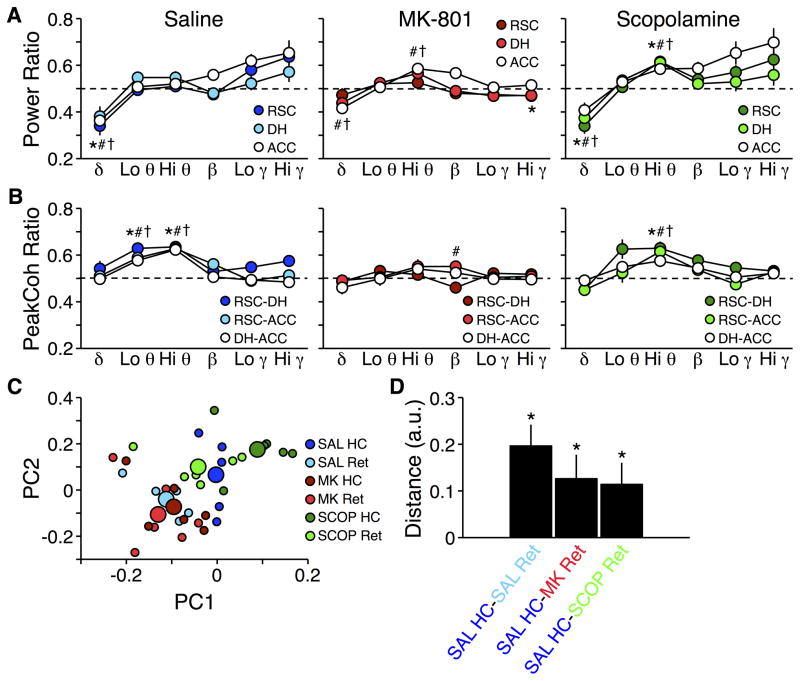
LFPs recorded during retrieval tests were compared to recordings made post-injection in the home cage to determine whether memory retrieval failure after MK-801 and SCOP were associated with similar or distinct patterns of power and peak coherence changes. During post-SAL retrieval, patterns of power and peak coherence across frequencies were similar to those seen in our previous experiment, during memory retrieval without any drug injection (Figure 1). There were no region by frequency or site-pair by frequency interactions for power or peak coherence, respectively (Fs10,90 < 1.55; ps > 0.13; Figure 4A–B, left). Nonetheless, delta power decreased in all three regions (F5,90 = 25.92; p < 0.0001) and both low and high theta peak coherence increased in all three site-pairs (F5,90 = 27.84; p < 0.0001).
Figure 4.
Power and peak coherence ratios during memory retrieval after drug injections. A) Power recorded during retrieval tests relative to post-injection recording sessions in the home cage. *, #, † p ≤ 0.01 vs. home cage for RSC, DH, and ACC power, respectively. B) Same as A for peak coherence. *, #, † p ≤ 0.01 vs. home cage for RSC-DH, RSC-ACC, and DH-ACC peak coherence, respectively. Dashed lines indicate no change from post-injection recording sessions in the home cage (ratio of 0.5). C) Plot of the first two principal components derived from recordings made in the home cage post-injection (HC) and during the retrieval test (Ret). Small symbols represent individuals in each session; large symbols represent the average for each session. D) Mean Euclidian distances between clusters in C. * Difference to hypothetical mean of 0 is non-negative and p < 0.01.
In contrast, MK-801 produced a region by frequency interaction in power (F10,90 = 2.17; p = 0.027; Figure 4A, center). DH and ACC delta power were decreased relative to the home cage, though to a lesser extent than post-SAL, but RSC delta power was no different than in the home cage. DH and ACC high theta power were increased, and any trend toward increased gamma power seen after SAL injection was abolished. Similar to SAL, there was no site-pair by frequency interaction for peak coherence (F10,90 = 1.81; p = 0.07; Figure 4B, center), but the post-SAL increases in theta peak coherence were eliminated.
The patterns of power and peak coherence during post-SCOP retrieval (Figure 4A–B, right) were qualitatively similar to those seen post-SAL. There were no region by frequency or site-pair by frequency interactions for power or peak coherence, respectively (Fs10,90 < 1.92; ps > 0.05; Figure 4A–B, left). As with SAL, delta power was consistently decreased across all regions, although high theta power was also consistently increased (F5,90 = 28.40; p < 0.0001). The retrieval-related increase in low theta peak coherence seen post-SAL was eliminated by SCOP, though the increase in high theta peak coherence remained (F5,90 = 20.25; p < 0.0001).
MK-801 and SCOP differentially affected baseline activity in the home cage. For state-space distance analysis, we therefore compared retrieval-related activity patterns from each test against activity recorded post-SAL in the home cage to determine drug effects relative to normal retrieval. This analysis confirmed that retrieval post-SAL was associated with patterns of LFPs that were distinct from in the home cage (t13 = 4.46; p < 0.001). Both drugs were also different from the post-SAL home cage baseline (MK-801: t13 = 3.37; p < 0.01; SCOP: t13 = 3.47; p < 0.01; Figure 4C–D), though these differences reflected distinct patterns of power and peak coherence changes across the network of structures we studied.
Discussion
With these experiments, we sought to define patterns of intra- and inter-regional oscillatory activity amongst a network of anatomically and functionally connected brain regions during encoding and retrieval of contextual memory, as well as effects on baseline and retrieval-related activity induced by amnestic drugs. Such oscillations are widely-recognized for contributing to mnemonic functions (Colgin, 2016; Corcoran et al., 2016). Consistent with this, we found that changes of LFP patterns were conserved across two different modes of contextual memory processing: memory encoding during exposure to a novel context and memory retrieval during a return to that context. Oscillatory activity across the network of structures studied was dominated by two key changes: decreased delta power and, consistent with our previous findings (Corcoran et al., 2016), increased theta peak coherence. These changes were not specific to a particular phase of memory processing, as they were similar during both encoding and retrieval. They were, however, robust, reproducible, and highly conserved across test sessions, and may thus provide a reliable readout of brain activity during exposure to a context that is different from the animal’s home cage.
The changes in oscillatory activity during memory retrieval were similar to the changes during encoding, but of lesser magnitude. It is possible that repeated exposure to the context could have eventually reduced LFP changes to zero, even though the memory of the context would have continued to be retrieved. This opens up the possibility that rather than encoding and retrieval, what we have observed here are activity changes reflecting novelty versus familiarity. Although we did not directly test that possibility here, one piece of evidence suggests that this is not the case. In our previous work, mice were fear conditioned and then exposed to the conditioning context for 8 consecutive days. Theta and gamma peak coherence in RSC-DH and RSC-ACC site pairs were unchanged from the first to the last of these extinction sessions (Corcoran et al., 2016). In that study, we did not perform the same comparison of LFPs in the home cage to LFPs in the conditioning context as we did here, but the lack of difference in coherence between the first and last return to the context suggests that repeated presentation of a stimulus does not eventually eliminate context-associated oscillatory activity within this network, and that habituation/familiarity alone cannot completely account for the decrease in LFP changes we observed between context encoding and memory retrieval sessions.
Oscillatory activity can be affected by a number of non-mnemonic processes, including arousal, valence, and locomotor activity, that could have contributed to the patterns of LFPs we observed here. Decreased delta power has been associated with increased arousal (Bódizs et al., 2001; Dang-Vu et al., 2008), but in our two tests, the causes of arousal were different (i.e., novelty vs. retrieval of memory for an aversive event). Emotional valence also cannot explain our findings, as the patterns of LFPs during encoding and retrieval of context memory were similar, despite the context having acquired a highly negative association as a result of fear conditioning between the two sessions. Locomotor activity has been correlated with changes in LFPs, especially in the theta range, but also cannot explain the patterns of oscillatory activity we recorded. As with valence, locomotor activity changed dramatically between encoding and retrieval sessions, but the overall pattern of LFPs was the same. Although we cannot completely rule potential contributions of arousal, valence, and locomotor activity to the changes in patterns of oscillatory activity we observed, at the same time these factors also cannot fully explain these changes. Thus, the broad trends we observed may provide a general signature of context processing, i.e., detection of being somewhere other than the home cage. In the network of brain regions selected for study here, we observed similar signatures of encoding and retrieval of context memory. Some cellular models of memory state that overlapping populations of cells are important for both encoding and retrieval (Cowansage et al., 2014; Liu et al., 2012); our data expand on this to suggest an analogous property at the systems level, such that there may also be overlapping network mechanisms for these processes
To test whether amnestic treatments target these conserved patterns of activity, we administered drugs that are known to affect memory processing. We chose MK-801 and scopolamine because, although they work through different neurotransmitter systems, they have similar and potent effects on behavior and mood (Costi et al., 2015; Drevets et al., 2013). As expected, SAL injection had no effect on baseline LFP patterns. In contrast, MK-801 produced region and site-pair specific effects, such as increased gamma power in RSC and DH, but not ACC, and increased delta and low theta peak coherence in DH-ACC, but not in RSC-DH or RSC-ACC. SCOP most robustly affected baseline power in the home cage, with a decrease in gamma and a trend toward increased delta, but had no effect on peak coherence.
Activity recorded during the post-SAL retrieval test was identical to that recorded in our earlier (drug-free) retrieval experiment, with network-wide decreases in delta power and increases in theta peak coherence. Interestingly, LFP changes during the SCOP test followed this pattern, which is the opposite of the SCOP-induced changes to baseline LFPs in the home cage. Thus, it is as if neural activity returned to baseline/home cage levels even though the mice were in the conditioning chamber; memory deficits caused by SCOP could be due to the drug preventing context-related LFP changes throughout this network. In contrast, MK-801 yielded retrieval-related LFPs that were markedly different from SAL. Patterns of changes in both power and peak coherence were flattened, particularly for theta peak coherence. Again, unlike SAL and SCOP, for which retrieval-related patterns of LFPs were conserved across all regions and site-pairs studied, MK-801 mainly produced effects that were unique to specific regions and site-pairs, such as preventing the test-related decrease in delta power only for RSC, and decreasing high gamma power only in DH. It is important to note that, besides the brain regions recorded here, systemic drug administration certainly affected LFPs in other brain regions important for memory processing, such as the amygdala. Thus, although unique changes in LFP patterns in the regions we studied may provide a useful readout for physiological effects of these drugs, their effects on behavior could have been mediated through activity changes in other regions.
Decreased freezing caused by the relatively low doses of these drugs used here was accompanied by distinct patterns of locomotor activity. After MK-801 injection, activity was similar to that seen prior to the foot shock on the conditioning day; after SCOP injection, activity was no different than after SAL injection, when the mice showed robust freezing responses. This difference in locomotor activity could be informative as to the nature of the memory deficits caused by the two drugs, as locomotor activity is inversely correlated with amount of exposure to a contextual stimulus. When first placed in a novel context, mice are motivated to explore and are thus highly active, but with repeated exposures to that context, locomotor activity habituates as the context becomes more familiar (McSweeney et al., 2002). The return to pre-conditioning levels of activity after MK-801 injection suggests that the mice did not recall that they had ever experienced the context before. In contrast, the loss of freezing after SCOP injection was not accompanied by a corresponding increase in locomotor activity, suggesting that the mice recognized the context as highly familiar but failed to recall the context-shock association. Thus, decreased freezing after drug administration was not due to hyperlocomotion, but was correlated with distinct effects on arousal and general levels of motor activity, indicative of fundamentally different forms of memory impairment.
At the beginning of this experiment, two outcomes were possible: the behavioral effects of these treatments would be mirrored in their effects on network activity, and both drugs would affect LFPs similarly; or, given that they work through different neurotransmitters, each drug would produce a unique pattern of changes in LFPs despite their similar behavioral effects. Our findings support the latter possibility: whereas drug-free context encoding and memory retrieval sessions were associated with homogeneous patterns of network activity, the drugs produced dissimilar patterns of changes, indicative of distinct mechanisms of action. That MK-801 more robustly affected peak coherence (i.e., long-range functional connectivity) during retrieval testing whereas SCOP mostly caused changes to baseline intra-regional power is consistent with the function of the neurotransmitter receptors they affect. Both glutamatergic and cholinergic receptors are important for generating local oscillatory activity (Pálhalmi et al., 2004; Shinozaki et al., 2016), but glutamate also plays a significant role in long-range excitatory transmission, which could drive coherent activity across structures
RSC, DH, and ACC comprise a part of the default mode network, whose activity is associated with cognitive functions including memory retrieval (Andrews-Hanna, 2012; Buckner et al., 2008; Spreng et al., 2009), and in which loss of functional connectivity is associated with a number of psychiatric disorders (Broyd et al., 2009). NMDA and muscarinic acetylcholine receptors have been implicated in many of the disorders associated with DMN dysfunction; here, we found changes in oscillatory activity within a homologous network in mice after disruption of these neurotransmitter systems. The only common effect of the two drugs was a network-wide failure to increase low theta peak coherence, which may point the way toward an electrophysiological “signature” for memory retrieval failure or general mnemonic dysfunction. In contrast, unique changes in network activity caused by these drugs may be related to affective and other non-mnemonic symptoms that are particular to different disorders associated with DMN dysfunction (e.g., hallucinations in schizophrenia; low mood in depression). Although not directly tested here, there is circumstantial evidence to support this possibility. In both humans (Costi et al., 2015; Drevets et al., 2013) and rodents (Autry et al., 2011; Corcoran et al., 2015; Navarria et al., 2015; Voleti et al., 2013), NMDA and muscarinic receptor antagonists have shown promise as rapid-acting antidepressants.
Multiple psychiatric disorders share overt behavioral symptoms despite being associated with dysfunction of different underlying neurotransmitter systems. Dysfunction of both glutamatergic and cholinergic signaling has been implicated in depression, schizophrenia, and other disorders characterized by cognitive and mnemonic deficits. Recently, there has been a push to study psychiatric disorders not according to symptomatology, but rather in terms of “disruptions of the normal-range operation of [the systems mediating normal brain function], with an emphasis on the mechanisms that serve to result in dysfunctions of varying degrees” (Cuthbert and Insel, 2013). In essence, this is a call to find common alterations in function, among the complex changes associated with different psychiatric disorders, which lead to similar behavioral, emotional, or cognitive symptoms. Understanding the role of network oscillations is especially important for this goal, given that several new therapeutic techniques, such as transcranial magnetic stimulation, transcranial direct current stimulation, and closed-loop stimulation, have profound direct and indirect effects on ongoing oscillatory activity in the brain (e.g., Marshall et al., 2006; Ngo et al., 2013). In this light, our current findings are relevant: they suggest that many disorders with distinct etiologies, but characterized by similar cognitive/mnemonic impairments, may be associated with relatively few common changes at the level of intra- and inter-regional network oscillatory activity. Targeting the underlying mechanisms of these shared changes may provide an avenue for novel treatments for common symptoms across psychiatric disorders.
Highlights.
Network oscillatory activity during memory processing was investigated
Encoding and retrieval of context memory yielded similar activity patterns
Drugs that block memory retrieval caused distinct changes to activity patterns
Acknowledgments
This work was supported by NIMH grant MH078064 and Dunbar Funds (JR), NUCATS grant UL1TR001422 and a Davee award (KAC). We would like to thank Dr. Leslie Kay for her assistance with data analysis. We declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-F, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bódizs R, Kántor S, Szabó G, Szûcs A, Eröss L, Halász P. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus. 2001;11:747–753. doi: 10.1002/hipo.1090. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–3. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Colgin LL. Rhythms of the hippocampal network. Nat Rev Neurosci. 2016;17:239–249. doi: 10.1038/nrn.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, et al. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci. 2011;31:11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Frick BJ, Radulovic J, Kay LM. Analysis of coherent activity between retrosplenial cortex, hippocampus, thalamus, and anterior cingulate cortex during retrieval of recent and remote context fear memory. Neurobiol Learn Mem. 2016;127:93–10. doi: 10.1016/j.nlm.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Leaderbrand K, Jovasevic V, Guedea AL, Kassam F, Radulovic J. Regulation of fear extinction versus other affective behaviors by discrete cortical scaffolding complexes associated with NR2B and PKA signaling. Trans Psychiat. 2015;5:e657. doi: 10.1038/tp.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Leaderbrand K, Radulovic J. Extinction of remotely acquired fear depends on an inhibitory NR2B/PKA pathway in the retrosplenial cortex. J Neurosci. 2013;33:19492–19498. doi: 10.1523/JNEUROSCI.3338-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costi S, Van Dam NT, Murrough JW. Current status of ketamine and related therapies for mood and anxiety disorders. Curr Behav Neurosci Rep. 2015;2:216–225. doi: 10.1007/s40473-015-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84:432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Pooviboonsuk P, Dalton JA, Lader MH. Differentiating the effects of centrally acting drugs on arousal and memory: an event-related potential study of scopolamine, lorazepam, and diphenhidramine. Psychopharm. 1998;135:27–36. doi: 10.1007/s002130050482. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseille M, Albouy G, Boly M, Darsaud A, et al. Spontaneous neural activity during human slow wave sleep. PNAS. 2008;105:15160–15165. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Zarate CA, Jr, Furey ML. Antidepressant effect of the muscarinic cholinergic receptor antagonist scopolamine: A review. Biol Psychiat. 2013;73:1156–1163. doi: 10.1016/j.biopsych.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Schwarz AJ. Large-scale functional connectivity networks in the rodent brain. NeuroImage. 2016;127:496–509. doi: 10.1016/j.neuroimage.2015.12.017. [DOI] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharm. 2009;34:932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiat. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Flint RW, Riccio DC. MK-801 induced retrieval, but not acquisition, deficits for passive avoidance conditioning. Pharm Biochem Behav. 2001;69:585–593. doi: 10.1016/s0091-3057(01)00565-2. [DOI] [PubMed] [Google Scholar]
- Honey GD, Honey RA, O’Loughlin C, Sharar SR, Kumaran D, Suckling J, et al. Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: an fMRI study. Cereb Cortex. 2005;15:749–759. doi: 10.1093/cercor/bhh176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Freeman WJ. Bidirectional processing in the olfactory-limbic axis during olfactory behavior. Behav Neurosci. 1998;112:541–553. doi: 10.1037//0735-7044.112.3.541. [DOI] [PubMed] [Google Scholar]
- Kim H. Default network activation during episodic and semantic memory retrieval: a selective meta-analytic comparison. Neuropsychologia. 2016;80:35–46. doi: 10.1016/j.neuropsychologia.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. PNAS. 2012;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S. Common processes may contribute to extinction and habituation. J Gen Psychol. 2002;129:364–400. doi: 10.1080/00221300209602103. [DOI] [PubMed] [Google Scholar]
- Navarria A, Wohleb ES, Voleti B, Ota KT, Dutheil S, Lepack AE, et al. Rapid antidepressant actions of scopolamine: role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis. 2015;82:254–261. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo H-VV, Martinetz T, Born J, Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Pálhalmi J, Paulsen O, Freund TF, Hájos N. Distinct properties of carbachol- and DHPG-induced network oscillations in hippocampal slices. Neuropharm. 2004;3:381–389. doi: 10.1016/j.neuropharm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Líbano D, Frederick DE, Egaña JI, Kay LM. The olfactory theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat. Front Behav Neurosci. 2014;8:214. doi: 10.3389/fnbeh.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannita WG, Maggi L, Rosadini G. Effects of scopolamine (0.25–0.75mg i.m.) on the quantitative EEG and the neuropsychological status of healthy volunteers. Neuropsychobiology. 1987;17:199–205. doi: 10.1159/000118365. [DOI] [PubMed] [Google Scholar]
- Sierakowiak A, Monnot C, Aski SN, Uppman M, Li T-Q, Damberg P, Brené S. Default mode network, motor network, dorsal and ventral basal ganglia networks in the rat brain: comparison to human networks using resting state-fMRI. PLoS ONE. 2015;10:e0120345. doi: 10.1371/journal.pone.0120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki R, Hojo Y, Makua H, Hashizume M, Murakoshi T. Kainate-induced network activity in the anterior cingulate cortex. Neurosci. 2016;14:20–29. doi: 10.1016/j.neuroscience.2016.03.025. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cog Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiat. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J, Stevens R, Robinson C. Effects of scopolamine on radial maze performance in rats. Physiol Behav. 1981;26:845–851. doi: 10.1016/0031-9384(81)90108-6. [DOI] [PubMed] [Google Scholar]
- Yu Y, Shen H, Zeng LL, Ma Q, Hu D. Convergent and divergent functional connectivity patterns in schizophrenia and depression. PLoS One. 2013;8:e68250. doi: 10.1371/journal.pone.0068250. [DOI] [PMC free article] [PubMed] [Google Scholar]